Fig. 10.1
Causal model whereby genes and environment affect brain systems responsible for expression of health behaviors, psychological traits and states, and neuroendocrine and autonomic functions that influence components of the body’s internal milieu in ways that, over time, lead to the development of CHD (Williams 2008)
As portrayed in this model, environmental social factors like social support, job strain, and stressful life events, and psychological factors like depression, hostility/anger, and anxiety do not influence the development and course of disease directly, but via effects on brain systems that regulate behaviors and the neuroendocrine/autonomic pathways that influence expression of biological phenotypes that are more proximately involved in the development and course of disease. As can also be appreciated from this model, one’s genetic makeup and the stressful environmental factors to which one is exposed can act both directly and via interactions between them to affect the brain systems that regulate behaviors, psychological traits/states, and autonomic/neuroendocrine systems that regulate expression of the biological systems that underlie the development and course of disease.
In order to understand how gene by environment interactions impact women’s health, therefore, it will be necessary to evaluate the influence of sex/gender on the pathways portrayed in this model. Based on extensive research showing that brain serotonin function is involved in the expression of negative emotions, aggressive behavior, alcohol and nicotine use, food consumption, and autonomic and neuroendocrine functions (reviewed in Williams 1994), my research group has evaluated the association between the functional promoter insertion/deletion polymorphism (5HTTLPR) on the serotonin transporter gene and an index of brain serotonin turnover—levels of the major serotonin metabolite 5-hydroxyindoleacetic acid (5HIAA) in cerebrospinal fluid (CSF)—in a sample of black and white men and women (Williams et al. 2003).
As shown in Fig. 10.2, both sex/gender and race moderated the association between 5HTTLPR genotype and CSF 5HIAA levels. In carriers of the more functional 5HTTLPR long (L) allele, men and women and blacks and whites had similar CSF 5HIAA levels, but in those homozygous for the less functional short (S) allele, however, there were marked differences. CSF 5HIAA levels were lower than other groups in men and whites with the SS genotype, but higher in women and blacks. These findings make a strong case that evaluation of associations between 5HTTLPR genotypes and phenotypes whose expression is influenced by brain serotonin needs to include evaluation of both sex and race as moderators of those associations. Depression is one such phenotype that has been the object of much research on the role of 5HTTLPR genotype.
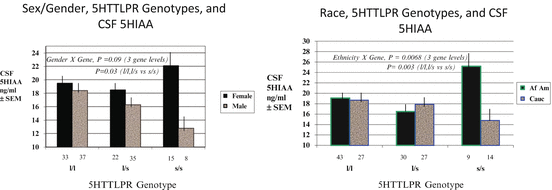

Fig. 10.2
Sex/gender and race moderation of 5HTTLPR association with CSF 5HIAA levels (Williams et al. 2003)
5HTTLPR and Depression
Based on earlier research finding that the 5HTTLPR S allele is associated with higher neuroticism scores (Lesch et al. 1996), there has been extensive research evaluating the association between 5HTTLPR genotype and depression. In a landmark study, Caspi et al. (2003) found that 5HTTLPR genotype was not directly associated with various measures of depression, but did interact significantly with stressful life events occurring during the preceding 5 years to predict depression, such that increasing stressful life events were associated with increasing depression levels, but only in carriers of the S allele, not those with the LL genotype. Despite this study’s promise that including consideration of the interaction between gene and environmental factors would increase our ability to identify genetic variants that contribute to the development and course of disease, a meta-analysis in 2009 (Risch et al. 2009) failed to confirm the 5HTTLPR × stress interaction effect on depression.
Variation in the measures of environmental stress and how sex was treated in the studies that failed to replicate the 5HTTLPR × stress interaction predicting depression could be responsible for the failures to replicate. In one study employing a general population sample, the S allele was associated with increased mental and physical distress in females but not males (Grabe et al. 2005). Even more strikingly documenting sex moderation of 5HTTLPR effects on depression, Sjoberg et al. (2006) found that females with the SS genotype were more likely to develop depression if exposed to family conflict, whereas in males the S allele was found to reduce depression in those exposed to family conflict.
These findings led my research group to evaluate sex/gender as a potential moderator of 5HTTLPR genotype effects on depressive symptoms in two independent samples, one exposed to a current life stressor (being primary caregiver for a relative with Alzheimer’s disease) and one exposed to a stressor during childhood (low SES indexed by low father’s education level) (Brummett et al. 2008a). As shown in Fig. 10.3, there was a significant sex/gender × 5HTTLPR × stress interaction in both groups. In both males and females there were lower depression symptom levels in the low stress groups—controls for caregivers and those with high father’s education—and there was no effect of 5HTTLPR genotype. In the high stress groups, however, depression symptom levels were higher and there was a significant effect of 5HTTLPR that differed as a function of sex/gender. In females depression levels were higher in those with the SS genotype; in contrast, among males, those with the LL genotype had higher depressive symptom levels.
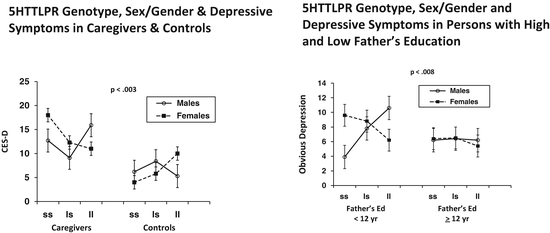

Fig. 10.3
5HTTLPR and depressive symptoms in males and females in groups with high current and childhood stress levels (Brummett et al. 2008a)
Inspection of Fig. 10.3 reveals another potentially interesting aspect of the gene by environment interaction effect on depressive symptoms in female caregivers and controls. Among those with the LL genotype CESD scores are similar in caregivers (10.8) and controls (10.0). In marked contrast, among women with the SS genotype caregivers have a CESD score (18) that is in the clinical range, but SS controls have a markedly low score of 4. Belsky et al. (2009) have called attention to this “plasticity” effect whereby the same genotype (5HTTLPR SS) can have adverse consequences (CESD scores in clinical range) in those exposed to a chronic stressor (caregiving), while in those with the same genotype who are not exposed to that stressor the effect is in the positive range (very low CESD score).
Considered along with similar findings in two prior studies (Grabe et al. 2005; Sjoberg et al. 2006) these remarkably consistent findings, showing 5HTTLPR effects on depressive symptom levels only in stressed persons in two independent groups—one with high current (caregiving) stress and one with high childhood (low SES) stress—that differ as a function of sex/gender make a strong case that attempts to find gene by environment interactions affecting negative mood, will need to consider sex/gender as a moderator of gene effects on mood in stressed persons. None of the negative studies in the Risch et al. (2009) meta-analysis evaluated the sex/gender × gene × stress interaction, and those that did include women did no more than covary sex/gender, which would not allow detection of the three-way interaction.
Another aspect of the Brummett et al. (2008a) study was the use of relatively objective indices of environmental stress—being the primary caregiver of a relative with Alzheimer’s disease or having a father with less than 12 years of education—that are less subject to recall bias than self-report measures of environmental stress exposure used in some studies. Karg et al. (2011) and Uher and McGuffin (2008) have noted the importance of using objective measures of environmental stress in studies attempting to identify gene × environmental effects.
In addition to evaluating sex/gender moderation of the 5HTTLPR by stress interaction effects on self-reports of depression (Brummett et al. 2008a), we have also evaluated sex/gender as a moderator of the association between 5HTTLPR genotype and negative moods engendered by acute increase of CNS serotonin levels induced by intravenous tryptophan infusion (Brummett et al. 2008b). In this study we also genotyped the rs25531 A > G SNP in 5HTTLPR that has been shown to modify the function of the L allele (Hu et al. 2006) such that L alleles with the rs25531 SNP A allele have high function, but those with the G allele have reduced function, to the same level as the S allele. This results in a triallelic genotype, such that high function LA is designated L′ and low function LG and S are designated S′.
As shown in Fig. 10.4, in males with the S′S′ genotype there was a small nonsignificant decrease in Depression-Dejection ratings following tryptophan infusion, but a marked increase (P < 0.02) in those with the L′L′ genotype. In contrast, among females, Depression-Dejection ratings did not change significantly in those with the L′L′ genotype, but increased significantly (P < 0.05) in those with the S′S′ genotype. Taken together with research showing effects of sex/gender on the association between 5HTTLPR and CSF 5HIAA (Williams et al. 2003) as well as negative moods in persons exposed to current or childhood life stress (Brummett et al. 2008a) that parallel those on the association between 5HTTLPR and negative moods engendered by acute increase in brain serotonin levels, these findings make a strong case that sex/gender must be considered in studies that aim to identify genetic variants that increase risk of depression in persons exposed to environmental stressors.
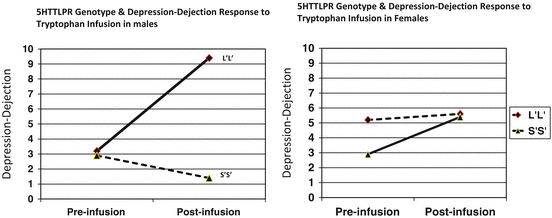

Fig. 10.4
Effect of tryptophan infusion on Depression-Dejection ratings in males and females with 5HTTLPR LL and SS genotypes (Brummett et al. 2008b)
The biological mechanisms accounting for these different effects of 5HTTLPR × stress interactions on depression in men compared to women remain to be identified. It is likely that effects of ovarian steroids on serotonin synthesis, serotonin transporter function, and also expression/sensitivity of pre- and postsynaptic serotonin receptors (Lu and Bethea 2002; Pecins-Thompson and Bethea 1999; Smith et al. 2004) are playing a role.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


