In other words, in animals other than humans, plasma low-density lipoprotein cholesterol (LDL-C) is constant around 25 mg/dl all their life; however, in humans, LDL-C increases up to four times in the first year after birth, and serum lipid itself plays a mandatory role in intimal hypertrophy and atherosclerosis formation (Fig. 3.1, right; Brown et al. 1980). In familial hypercholesterolemia, LDL-C increases two to ten times higher than normal human plasma LDL-C levels. There are no sex differences in LDL levels, and high-density lipoprotein cholesterol (HDL-C) levels are approximately 10 mg/dl higher in females than in males. Estrogen promotes hepatic apolipoprotein A-1 synthesis and inhibits hepatic triacylglycerol (TG) lipase activation. LDL is taken up from the circulation due to accelerated very-low-density lipoprotein (VLDL) production and an increased number of hepatocyte LDL receptors. LDL oxidization is inhibited and coronary risk factor lipoprotein(a) (Lp(a)) is also lowered by estrogen (Brinton 1996; Su et al. 1998). Menopause results in negation of estrogenic effects, leading to lipid changes, and explains approximately 50 % of the postmenopausal cases of rapid atherosclerotic progression (Holm et al. 1999). This drastic change of lipid profile in postmenopausal women reflects the gender differences and change after its 50 years of incidence of ischemic cardiovascular changes (Fig. 3.1, left).
3.2.3 Effects of Estrogen on Nitric Oxide Synthase (NOS)
Most of the remaining anti-atherosclerotic effects of estrogen are believed to arise from direct effects on the vascular wall and, in particular, the endothelium. Estradiol (E2) provides antioxidant action. We discovered that E2 has biphasic action that makes NOS activation and NO production receptor dependent in bovine aortic endothelial cell (BAEC) and human umbilical vein endothelial cell (HUVEC) (Hayashi et al. 1995a). This decreases NOS activation and NO production at pharmacological concentrations (Hayashi et al. 2000a). This suggests that the endothelial nitric oxide synthase (eNOS) sites of E2 action are estrogen response element half-palindromic motifs and AP1 and SP1 regions (Simoncini et al. 2002). E2 inhibits destabilization of endothelial nitric oxide synthase messenger ribonucleic acid (eNOS mRNA) in the presence of tumor necrosis factor-alpha (TNF-α) (Sumi et al. 2001). E2 also increases the number of caveolae, which are eNOS sites of action. Estrogen action diverges at eNOS/NO. T cell- and macrophage-derived inducible nitric oxide synthase (iNOS) was observed around the necrotic foci (soft, friable plaque with abundant inflammatory cells) in the aorta of domestic rabbits and advanced coronary atherosclerotic lesions in humans (Esaki et al. 1997). E2 inhibited macrophage system cell J774 and neutrophil iNOS (Hayashi et al. 1998). Estrogen also has acute effects mediated via eNOS phosphorylation, as well as cell membrane estrogen receptor alpha (ERα) and Akt/PKB, although independent of intranuclear receptors (Hisamoto et al. 2001; Haynes et al. 2000). It also influences atherosclerotic plaque formation (including leukocyte adhesion and migration), angiogenesis/vascularization, and the myocardium. There is a high homology of ERβ with ERα DNA-binding domains, and previous reports have suggested that mediation occurs via ERβ as well as ERα. In dietary cholesterol-loaded rabbits, ERα was observed in the endothelium, while ERβ was observed in the endothelium and around the necrotic foci. When dietary cholesterol loading was conducted on male rabbits and female rabbits which had not undergone ovariectomy, atherosclerotic lesion formation in female rabbits was milder than in male rabbits (Hayashi et al. 2000a, 1995b). With regard to the NO basal secretion observed in extracted blood vessels, reduced NO secretion in females preceded lesion formation. In males, NO basal secretion was originally low. High levels of basal NO secretion in females work defensively against dietary cholesterol-loading stimuli, leading to the hypothesis that E2 has anti-atherosclerotic effects mediated via NO (Hayashi et al. 2000a, 1995b). The effect of estrogen on EDHF was also suggested (Sakuma et al. 2002).
3.2.4 SERM
Estrogen acts on the reproductive and coagulation systems in addition to the cardiovascular system, and hormone replacement therapy (HRT) side effects have obstructed its popularization (Hlatky et al. 2002; Writing Group for the Women’s Health Initiative Investigators 2002). Selective estrogen receptor modulators (SERM) were developed taking this into consideration, leading to modulators such as raloxifene being made available. NO-dependent ameliorative effects on endothelial function were also reported, and it is possible that this may solve many issues related to HRT (Ettinger et al. 1999; Wenger et al. 2002).
3.3 Effects of Estrogen on Blood Vessels: Clinical Results
In the arteries of young females, flow-mediated dilation (FMD) fluctuates in tandem with estrus cycles due to estrogen, and FMD decreases after menopause (Hashimoto et al. 1995). When estriol (E3) was administered to elderly females who are more than 25 years postmenopause, however, improved FMD and increased plasma nitrogen oxide (NOx) and cyclic guanosine monophosphate (cGMP) were observed after 30 weeks (Hayashi et al. 2000c).
We created guidelines for HRT for postmenopausal Japanese patients, based on clinical research presided over by the Japanese Ministry of Health, Labour and Welfare as a Longevity Science Research Project (1998–2003) (Watanabe et al. 2004). When 305 women (246 of whom were undergoing HRT, mean age of 63 years) were administered low-dose conjugated estrogen, E3 pharmaceuticals, or E3 patches and observed for an average of 3 years, genital bleeding was observed in 20 % and mastalgia in 16 %. There does, however, appear to be a racial difference with Western patients as no ischemic heart disease, cerebrovascular accidents, or uterine, ovarian, or mammary tumors were observed (data were reported in Japanese only).
In general, HDL concentration is approximately 10 mg/dl higher in females than males, and this difference shrinks following menopause. Within approximately 2 years, HDL decreases by 5–7 %, and TC and LDL increase by approximately 10 %.
Arterial thrombosis is also affected by estrogen in a dimorphic pattern. NO-mediated antithrombotic action occurs at low concentrations, while thrombogenic steroid actions (similar to the contraceptive pill) occur at high concentrations (Battaglioli and Martinelli 2007; Shulman 2011). Deep vein thrombosis is also a potential adverse event. In the Heart and Estrogen Replacement Study (HERS) that targeted patients affected by cardiovascular disease, the most common reason for discontinuation of medication was venous thrombosis in the initial period of estrogen administration (Hlatky et al. 2002; Shulman 2011). The incidence frequency of coagulation factor gene mutations (factor V Leiden mutations) in Asian races is lower than in Western races, and venous thrombosis is uncommon (Ridker et al. 1997). In South Korea, HRT is recommended even now.
3.4 Estrogen and Cellular Aging
3.4.1 Estrogen and Cellular Aging Through NO
Estrogen affects vascular and cellular aging, which is involved in the sex differences apparent in average life expectancy.
Cellular aging markers in cultured endothelium: SA-β-gal positive = senescent cell count was reduced with physiological concentrations of estrogen. This action suggests mediation via NO, as this is inhibited by ER and NOS inhibitor nitro-l-arginine methyl ester (L-NAME) (Fig. 3.2, right; Hayashi et al. 2006a). Even at physiological estrogen concentrations, 70 % of atherosclerotic changes in dietary cholesterol-loaded domestic rabbits were inhibited, albeit without changes in serum lipid levels (Hayashi et al. 2000a). In atherosclerosis prevention, estrogen acts via aliquots of NO and exhibits anti-arteriosclerotic effects in proportion to serum estrogen concentration and tissue concentrations of the NO reaction product cGMP (Fig. 3.2, left). The mechanism of maintaining NO effects on atherosclerosis or vascular aging is not due to suppression of the citrulline–arginine system, but rather that of arginase II (Fig. 3.3; Hayashi et al. 2006b). Estrogen thus increases the bioavailability of NO in progressive atherosclerotic vascular disease.
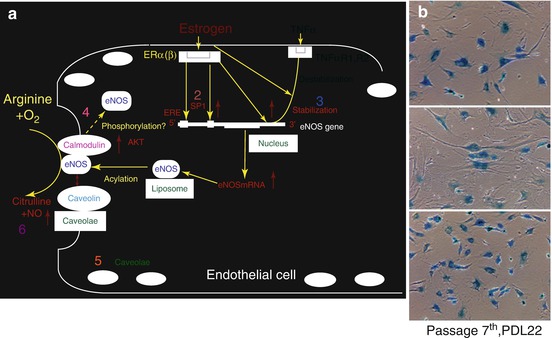
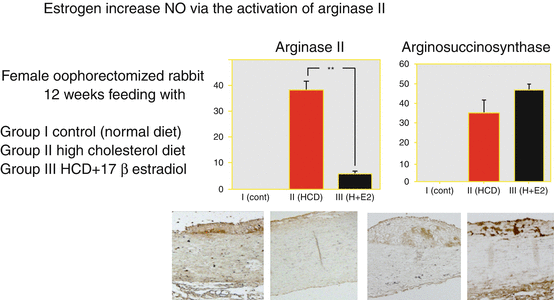

Fig. 3.2
Mechanism of NO release enhancement by estrogen and the effect of aging of estrogen. (a) Left: Under the normal homeostasis condition, endothelial NO is synthesized from l-arginine and oxygen in a reaction catalyzed by eNOS. Estrogen activates eNOS expression through the PI3-K/Akt pathway and/or direct effect via promoter or stabilization of eNOS mRNA. They may also inactivate NADPH oxidase. NO shows antiapoptotic effects and suppresses ROS production by scavenging directly or preventing NADPH. However, with the normal condition of endothelium, the balance between the antioxidant effects of NO and ROS production may be kept well. Solid lines represent positive regulatory pathways. Dotted lines represent negative regulatory pathways. 1. Receptor mediated gender difference (Cited from Hayashi et al. 1992). 2. Promoter (Cited from Hayashi et al. 1995a). 3. mRNAstablization (Cited from Sumi et al. 2001). 4. Calmodulin (Cited from Hisamoto et al. 2001). 5. Caveolae (Cited from Hayashi et al. 1995a, 2000a). 6. Superoxide anion (Cited from Hayashi et al. 2000b). 7. EDHF (Sakuma et al. 2002). 8, 9 Phosphorylation (Cited from Haynes et al. 2000, Hayashi et al. 2006a). (b) Right: Effect of estrogen on cellular senescence. Representative photographs of SA-β-gal staining in control, 10-8 M E2-treated, and 10-8M E2- and 10-4M L-NAME-treated cells. Note that treatment with E2 decreased the number of SA-β-gal-positive cells, which was prevented by further treatment with L-NAME. Cells were used in PDL 22 at passage 7 (Cited from Hayashi et al. 2006a). TNFarufa tumor necrosing factor arufa, ERE etrogen response element (a human transcription factor), SP1 Specificity Protein 1 (a human transcription factor)

Fig. 3.3
Modulating role of estradiol on arginase II and arginosuccinosynthase expression in hyperlipidemic rabbits as an atheroprotective mechanism. Upper: Distribution of arginase II in atherosclerotic aortas. Immunohistochemical analysis with anti-arginase II antibody of thoracic aortas of NZW rabbits from the different groups. **, P < 0.01. Lower: Immunohistochemical analysis with anti-arginase II [lower left: group II and lower middle left: group III] monoclonal antibodies and the anti-argininosuccinate synthetase monoclonal antibody [lower middle right: group II and lower right: group III] of the thoracic aortas of NZW rabbits from the atherosclerotic group (original magnification, ×100, scale bars 25 μm; Cited from Hayashi et al. 2006b)
3.4.2 Age-Related Fluctuations in Sex Hormones
The serum concentrations of sex hormones change dramatically over the course of one’s lifetime. Adrenal androgens such as dehydroepiandrosterone (DHEA) decrease with age from puberty onward in both males and females (Hinson et al. 2003). Estrogen decreases dramatically in females after menopause and from then on is mainly composed of estrone (E1) from peripheral tissues due to the action of aromatase. Estrogen in males remains at a fixed concentration throughout their entire lifetime and is slightly higher than postmenopausal females. After DHEA was converted into E2 by aromatase, it exerts anti-atherosclerotic effects via NO (Hayashi et al. 2000b).
3.5 Sex Differences in Geriatric Disease and Coronary Risk Factors
Most diseases in elderly patients have atypical symptoms and signs, e.g., asymptomatic angina pectoris in elderly diabetic individuals. Research is also progressing regarding sex differences and their relation to sex hormones for each of these diseases.
3.5.1 Dyslipidemia
Dyslipidemia significantly influences cardiovascular disease sex differences in postmenopausal and elderly patients. High serum LDL cholesterol and low serum HDL cholesterol are also independent risk factors for ischemic heart disease (IHD), cortical branch CVAs, and arteriosclerosis obliterans (ASO) in elderly patients (Aronow 2002). The influence of statins did not live up to the expectations in large-scale clinical trials (Prosper, HPS, ASCOT-LLA) that included Western late elderly subjects (Heart Protection Study Collaborative Group 2002; Sever et al. 2003; Shepherd et al. 2002). Although effects were not seen in women in the Anglo-Scandinavian Cardiac Outcomes Trial – Lipid Lowering Arm (ASCOT-LLA), sex differences are still possible as the study only involved a small number of cases (Shepherd et al. 2002). Meanwhile, our investigation of 4,014 patients (MHLW-funded research group headed by the author) revealed reliable treatment outcomes in women with concomitant diabetes (Hayashi et al. 2008).
In familial hyperlipidemia, there are no sex differences in serum lipid levels, and a sex difference is observed for the age of cardiovascular disease onset (approximately 20 years) (Oosterveer et al. 2009). There is also hardly any fluctuation in serum lipid levels due to E2 administration in dietary cholesterol-loaded animals (Hayashi et al. 2000a). The pleiotropic effect, which is recognized with statins, is also observed with estrogen. More doctors are offering guidance regarding other risk factors appropriate to age and sex.
Human TC levels are lowest in both males and females before 20 years of age and are said to increase with age (Brown et al. 1980; Sekimoto et al. 1983). An annual, in-class cohort study conducted on 20,000 screening examinees revealed trend of increasing TC levels until around 75 years of age (Sekimoto et al. 1983). Cases with TC values of 200 mg/dl or higher were relatively elderly and increased by twofold from 1980 to 1990; approximately 25 % of males and 45 % of females showed plasma TC levels higher than 220 mg/dl (JAPAN Guideline for Diagnosis and Prevention of Atherosclerotic Cardiovascular Diseases 2007). For plasma LDL cholesterol levels, almost the same situation occurred (Kuzuya and Shimokata 2002). Regarding postmenopausal female hyperlipidemia patients, research revealed that coronary heart disease primary preventative effects were observed in Japanese patients with a mean TC of 240 mg/dl, 60 % of whom were females (mega study) (Mizuno et al. 2008). The Japan Lipid Intervention Trial (J-LIT) showed a dramatic increase in cardiovascular disease incidence in females with dyslipidemia due to concomitant IGT abnormalities and smoking (Yokoyama et al. 2007). Use of HRT to prevent ischemic heart disease (IHD) decreased after the results of the Women’s Health Initiative (WHI), but HRT has been reported to have an inhibitory effect on calcification that occurs in end-stage atherosclerosis (Writing Group for the Women’s Health Initiative Investigators 2002; Manson et al. 2007).
E2 patches are considered very promising for HRT as they bypass hepatic metabolism and do not elevate TG. E3 does not cause serum lipid fluctuations in females directly after menopause, and HDL increases from 6 months after administration in patients with a mean age of 80 years (Hayashi et al. 2000c).
3.5.2 Hypertension
The Framingham study found that the incidence of cardiovascular disease complications increased in both males and females in proportion to hypertension severity (blood pressure) (Engberding and Wenger 2008). Hypertension is a risk factor for renal failure, cerebral infarction, and IHD. Hypertensive cardiovascular disease onset frequency and morbidity rate are both high in females. Senescent elastic aortic atherosclerosis leads to systolic hypertension, and this elastic aortic atherosclerosis leads to decreased diastolic blood pressure, reduced coronary blood flow, and cerebral ischemia (Dao et al. 2005). Systolic hypertension also contributes to myocardial infarction and cerebral infarction onset.
Hypertension onset occurs later in females than in males and often happens after menopause. Hypertension is often complicated by cardiac failure from age 70 onward. There is a debate regarding whether hypertension contributes to estrogen shortage. Animal experiments and cell biological studies have reported that estrogen shortage causes (1) changes related to renal diuresis, (2) expression of angiotensin II receptors and increased secretion of aldosterone, and (3) impaired secretion of arteriolar EDHF and NO (Nawate et al. 2005). On the other hand, it has also been reported that there is no difference in blood pressure fluctuation caused by menopause, HRT, hysterectomy, or before and after menopause (Taddei 2009).
3.5.3 Diabetes Mellitus
As there are multiple branches of the coronary artery, in diabetic angiopathy, diffuse long stenotic lesions are common. There is little morphological change or lipid accumulation. Diabetes is the greatest risk factor for ASO, and elderly females with diabetes are also at high risk for cardiovascular disease. Estrogen prevents vascular endothelial dysfunction caused by hyperglycemia by activating the rate-limiting enzyme GTPCHI of NOS coenzyme BH4 (Miyazaki-Akita et al. 2007). This is anticipated to aid in the prevention of diabetic cardiovascular diseases. As high-dose estrogen causes hyperglycemia through glucocorticoid action, HRT is contraindicated in severe diabetic cases. In apoE KO mice, estrogen inhibited streptozotocin-induced diabetes and progression of atherosclerosis. Diabetes onset was later in normal female rats than in rats that underwent ovariectomy (Tse et al. 1999). End-stage renal failure is often caused by hypertension and nephritis in males, and there is no sex difference in diabetes origin. Our investigation of 4,014 diabetic patients including 1,016 late elderly diabetic patients older than 75 years (MHLW-funded research group headed by the author) revealed that HDL-C levels are important for the prevention of ischemic heart disease and cerebrovascular attack (stroke) and that insulin treatment might require close attention because of the risk of hypoglycemia in elderly (Hayashi et al. 2009, 2011, 2013).
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


