Table 47.1
Lesions identified as source of lower gastrointestinal bleeding in patients after LVAD implantation, modified from Draper et al. [4]
Source | Frequency (%) |
|---|---|
Angiodysplasia | 29 |
Gastritis | 22 |
Ulcer | 13 |
Diverticulitis | 6 |
Polyp | 5 |
Colitis | 3 |
Other/unkown | 22 |
47.1 Diagnostics
The cause of bleeding is found in approximately 75% of the patients. In case of a suspicious upper GIB, the best diagnostic tool is an upper endoscopy, for lower GIB a colonoscopy. If the upper or lower endoscopy is not successful, a capsule endoscopy is a less traumatic method. The diagnostic yield of capsule endoscopy has been superior in the diagnosis of small bowel disease compared to small bowel series, computerized tomography, or push enteroscopy. The results of the capsule study may indicate the further need for therapeutic intervention by a double balloon endoscopy [7]. To detect the source of GIB by angiography, the bleeding has to be more than 0.5 ml/min and for tagged red blood cell scan for more than 0.1 ml/min. The yield of different diagnostic tools is evaluated by Kushnir et al. in 44 episodes of GIB in LVAD patients, ◘ Fig. 47.2 [8].
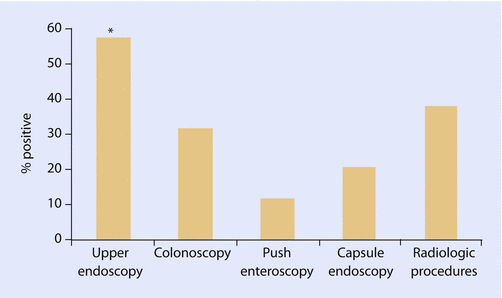

Fig. 47.2
Diagnostic yield of endoscopic and radiologic procedures performed for the evaluation of gastrointestinal bleeding in patients with LVADs. Upper endoscopy had the highest yield for a source of bleeding, significantly higher than that seen with colonoscopy or enteroscopy. The number of capsule endoscopy (5) and radiologic procedures (8) performed was limited [8]
47.2 Risk Factors
One reason for the high rate of GIB in patients after continuous-flow LVAD implantation is a dual therapy with vitamin K antagonist and a platelet aggregation inhibitor. However, the higher rate of GIB in LVAD patients than in comparable anticoagulated patients without an LVAD can be explained by additional coagulation disorder in LVAD patients. Importantly, patients with a continuous-flow LVAD develop an acquired von Willebrand syndrome due to the shear stress of the pump and thus show a dysfunction of the primary hemostasis [9, 10]. Additionally, an impaired platelet function was detected in these patients by Klovaite et al. [11]. Furthermore several risk factors were identified to influence the GIB. In majority of the studies, age represents in a multivariate analysis a significant risk factor for GIB [1, 12]. Therefore older patients with an age > 65 years show a 1.5-fold higher risk for bleeding [3]. Other studies identified a history of GIB before LVAD implantation as a significant risk factor [2, 13].
Decreased pulsatility in continuous-flow LVADs may also contribute to GIB as lower tertiles of pulsatility index subsets demonstrated in a study from Wever-Pizon et al. as a significant risk factor for GIB [2]. Renal dysfunction reflected in elevated creatinine impairs platelet function; therefore an elevated creatinine was observed in the group of patients with GIB [1].
Goldstein found that patients with GIB after LVAD implantation had a significant higher body mass index, more frequently diabetes and ischemic etiology of heart failure which were further confirmed in other series [1, 12]. Adipose tissue is a complex highly secretory endocrine organ that can regulate insulin sensitivity and inflammation and releases among others vascular endothelial growth factor (VEGT), TNF-α, and inflammatory cytokines leading to impaired vasoregulation [14–16]. However, the correlation of the development of vascular changes in terms of angiodysplasia and the different flow pattern in continuous-flow LVADs are not completely defined.
47.3 Therapy
The therapy of a GIB primarily consists of the analysis of blood and coagulation parameter with subsequent correction of anemia and potential excessive anticoagulation. The minimal hemoglobin level should be more than 6 g/dL. The transfusion of one unit of packed red blood cell concentrate increases the hemoglobin level of 1.0–1.5 g/dL. The administration of fresh frozen plasma and coagulation factors is recommended. Due to the chronic antiplatelet therapy, application of platelet concentrates should be considered. Secondly, detection of the source of bleeding with its possible elimination is of a paramount importance. An algorithm for the evaluation and treatment of GIB is provided by Suarez et al., ◘ Fig. 47.3 [17].
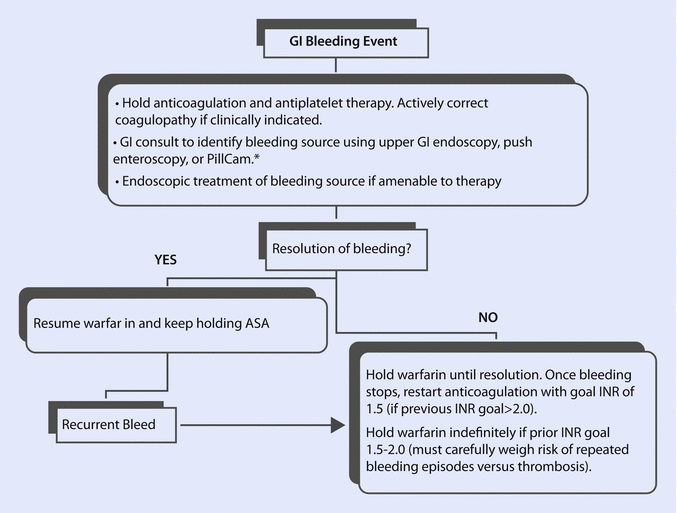

Fig. 47.3
Algorithm for gastrointestinal bleeding in LVAD patients by Suarez et al. *There are no data indicating that endoscopy or the PillCam are beneficial in management of GIB early after LVAD implantation when pre-LVAD endoscopy showed no bleeding source [17]
The anticoagulation should resume with vitamin K antagonist. A platelet inhibitor may be paused. In recurrent GIB, high urgency or UNOS 1A listing for heart transplantation should be considered. A surgical solution with partial resection of the intestine is a high-risk procedure in these patients and should be reserved as the last resort. The recurrence of a GIB was described in up to 43% of the patients with a GIB [2, 13, 18, 19]. Aggarwal et al. found that 60% of the patients had a recurrence of bleeding from the same anatomic site [18].
Reports on pharmacological therapy of GIB are typically isolated case reports or case series. The effectiveness of estrogen and progesterone is not clarified [20], and a multicenter, randomized, placebo-controlled study failed to show an effect of hormonal therapy in the prevention of rebleeding from gastrointestinal angiodysplasia [21]. Medical treatment with subcutaneous octreotide of 50 μg twice a day is described by Junquera et al. as a potential treatment in preventing rebleeding from gastrointestinal angiodysplasia. The somatostatin analogue reduces the portal venous pressure and decreases the splanchnic blood flow. The side effects were diarrhea and abdominal pain [21]. Thalidomide was used due to the anti-angiogenetic effect in patients with GIB. To date, a randomized study supporting this approach is missing [22].
Another future therapeutic strategy represents a prevention of excessive degradation of HMW-VWF multimers by partial inhibition of VWF-ADAMTS13 interactions with a use of an anti-VWF monoclonal antibody published by Rauch et al. [23]. Recently, a novel approach to ADAMTS13 inhibition was proposed in experimental model by administration of doxycycline [24].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


