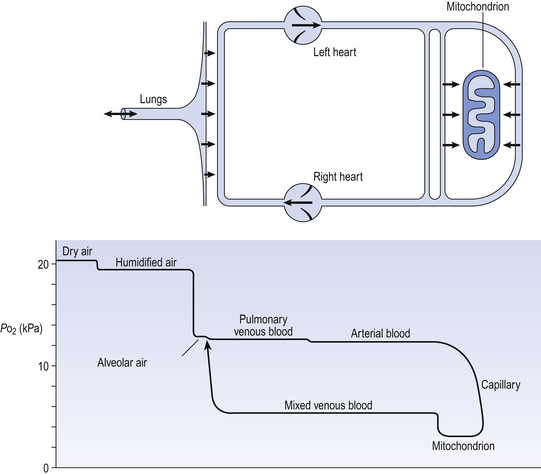
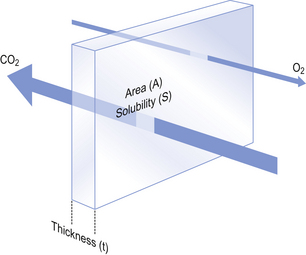
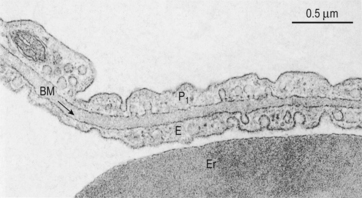
6 In Chapter 5 we described the way in which ventilation of different regions of the lung results in different compositions of gas in the alveoli. In understanding the next step in the journey from air to blood or blood to air it is important to differentiate clearly between the amount of a gas present in a mixture and the fraction or concentration of that gas, frequently expressed as partial pressure (P). This concept is important because it is only the difference in partial pressure that drives diffusion from one place to another. To use an analogy: the pressure across the enormous lock gates holding the Atlantic Ocean out of a dock in which the water is 10 cm lower than the ocean is the same as that across the walls of a child’s paddling pool containing water 10 cm deep. The amounts of water either side (or gas in the case of the lung) having nothing to do with the motive force (driving pressure): the difference in depth of water (or difference in partial pressure of gas) provides the motive force. The partial pressure of a gas depends on its concentration, and so the partial pressure gradient across a membrane depends on the difference in concentration on either side. It is diffusion alone that transports gas between air and blood at the lungs, and between blood and cells at the tissues, and the circulation of blood effectively links the two sites. In the case of O2 (Fig. 6.1) we can see that diffusion at the lungs is sufficient to reduce to virtually zero any difference in partial pressure (concentration) between alveolar air and pulmonary blood, whereas at the tissues a large difference in partial pressure between arterial blood and the mitochondria ensures a vigorous flow into these organelles of oxidative metabolism. The single step of O2 from air to blood in Figure 6.1 is in fact a series of steps through components which are in series and which will be considered in detail below. The important thing to remember is that they are in series (i.e. one after another), like the segments of a hosepipe made up of a number of bits joined together. Constricting one segment reduces flow in all. The lungs have such an enormous capacity for diffusion that there is some doubt in many diseases (e.g. emphysema) as to what part the impairment of diffusion plays in producing the characteristic hypoxia of the disease. In many cases a diffusional component can only be demonstrated when the patient is exercising. The destruction of the architecture of the respiratory regions in emphysema (Fig. 4.16) must impair diffusion, but the effect on distribution of ventilation and blood flow must be at least as important. Other diseases (e.g. interstitial pulmonary fibrosis) which produce profound thickening of the respiratory membranes reduce the lungs’ capacity for diffusion to one-sixth of normal, and it is difficult to assume that this does not affect their function. The movement of gas into and out the blood is described by Fick’s Law (Appendix, p. 155), which describes the rate of diffusion of a gas across a membrane as follows: where A is the area of the membrane available for diffusion. S is the solubility of the gas in the membrane, ΔC is the concentration gradient – brought about by the differences in concentration (or partial pressure) either side of the membrane – t is the thickness of the membrane and MW is the molecular weight of the gas (Fig. 6.2). 1. the reaction releasing CO2 from blood is relatively slow 2. the concentration gradient driving CO2 from blood to alveolar air is only 0.8 kPa, whereas that driving O2 in the opposite direction is 8 kPa. Although there are situations when the lung can be so damaged as to restrict the diffusion of CO2 it is usually O2 that is first affected by diffusional difficulties. The rate of removal of CO2 is governed primarily by alveolar ventilation (chapter 5; p. 62 and p.83 of this chapter). In Figure 6.1 there are three places where diffusion is the major transport mechanism and where this transport can effectively be impeded by failure of diffusion: As this is a textbook on the respiratory system we will concentrate on the first two. The anatomical sites of these two sources of impedance to diffusion are shown in Figure 6.3.
GAS EXCHANGE BETWEEN AIR AND BLOOD
DIFFUSION
The path from air to tissue
Lung disease and diffusion
Fick’s Law of Diffusion
GAS EXCHANGE BETWEEN AIR AND BLOOD: DIFFUSION