Drug or strategy
Major inference with respect to type 1 cardiorenal syndrome
High dose loop diuretics
↑ AKI, no reduction in rehospitalization or death [2]
Continuous infusions of loop diuretics
↑ AKI, no reduction in rehospitalization or death [2]
Low-dose dopamine infusion (stimulate D1>>β1 receptors)
Improved urine output, but no ∆ AKI, no reduction in rehospitalization or death [3]
Nesiritide (recombinant B-type natriuretic peptide) at any dose or infusion protocol
No ∆ AKI, no reduction in rehospitalization or death [4]
Dobutamine (β1 agonist)
No high quality trials with CRS outcomes
Milrinone (phosphodiesterase 3 inhibitor)
No high quality trials with CRS outcomes
Levosimendan (myocardial calcium sensitizer)
No high quality trials with CRS outcomes, however, ↓AKI after cardiac surgery [5]
Rolofylline (selective adenosine A1 receptor antagonist)
No ∆ AKI, no reduction in rehospitalization or death
Tolvaptan (arginine vasopressin receptor antagonist)
No ∆ AKI, no reduction in rehospitalization or death [6]
Programmatic use of invasive hemodynamic monitoring
No ∆ AKI, no reduction in rehospitalization or death [7]
Ultrafiltration for diuretic resistance before AKI develops
↓Weight, reduction in rehospitalization and death [8]
Ultrafiltration after AKI develops
No ∆ weight, no reduction in rehospitalization or death [9]
The Acute Dialysis Quality Initiative has recently published a consensus on the pathophysiology of type I CRS [12]. This syndrome has established risk predictors including hypertension, elevated jugular venous pressure, pulmonary congestion, and reduced renal filtration function at baseline (Fig. 18.1). A proposed approach to subset patients according to hemodynamic and pathophysiologic mechanisms is shown in Fig. 18.2. Because it is believed that a very narrow therapeutic window of intravascular volume management exists for the kidney in the setting of ADHF (Fig. 18.3), it follows that some attempt based on clinical exam, chest-x-ray, noninvasive hemodynamic measures, and even invasive pressure measures are needed to adequate select a patient for a specific therapy. For patients who have intact perfusion and little or no congestion, then minimal diuresis and adjustment to baseline medications is a reasonable strategy. For those with impaired perfusion but still little or no congestion, in the absence of ischemic cardiomyopathy, use of a renal-dose adjusted inotropic agent such as milrinone or levosimendan could be a reasonable pathway. If there is intact perfusion but considerable congestion, then use of vasodilator agents such as nitroglycerin or nesiritide could be entertained. Finally, those with impaired perfusion and pulmonary/systemic congestion and the highest risk for type 1 CRS and inpatient mortality, combined use of diuretics and inodilators, and possibly early continuous renal replacement therapy could be considered. It is important to understand in each of these four scenarios, none of the therapies above have been specifically tested in that subset in an adequately powered, randomized, placebo controlled randomized trial. Hence, novel agents should have the advantage of collective hindsight and be tested in specific phenotypic patients at risk for type 1 CRS in order to give the most valid treatment inferences and help move the field forward.
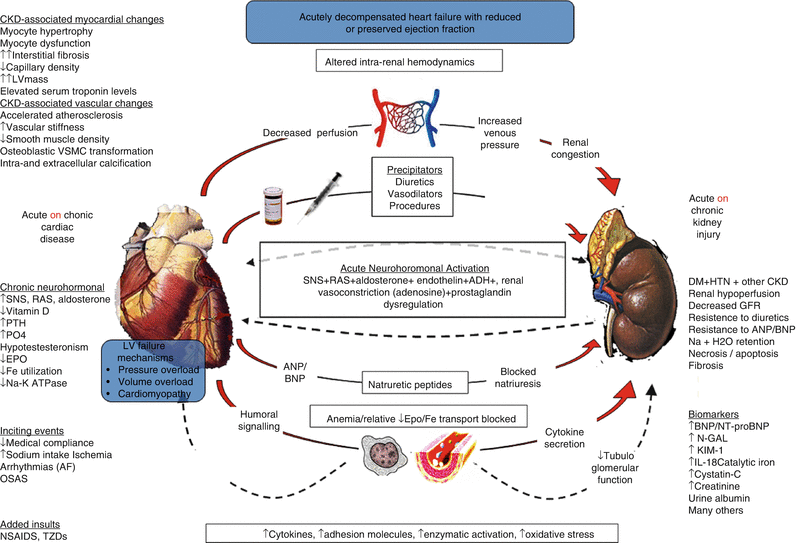
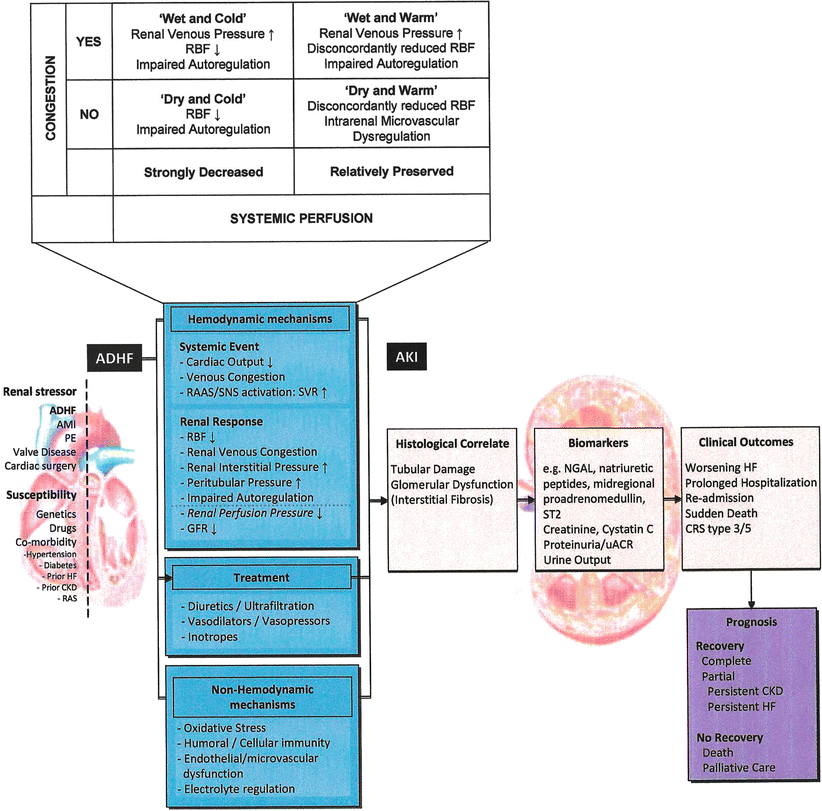
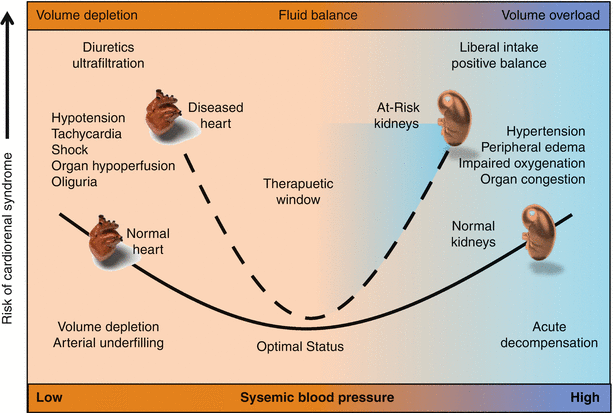

Fig. 18.1
Complicated pathogenesis of acute type 1 CRS

Fig. 18.2
Hemodynamic subsets and their relationship to the pathophysiology of type 1 CRS

Fig. 18.3
Narrow therapeutic window for intravascular volume management in type 1 CRS
Conventional Therapies for Acutely Decompensated Heart Failure
The National Institutes of Health, National Heart Lung and Blood Institute Heart Failure Clinical Research Network has sponsored three randomized trials with specific attention on both the cardiac and renal implications of patients admitted with ADHF [3]. The DOSE trial found no benefit of high-dose or continuous infusions of loop diuretics, in fact, there were higher rates of AKI with those approaches [2]. The CARRESS-HF trial found no benefit for ultrafiltration in patients once type 1 CRS had fully developed, and in fact, since the rate of fluid removal exceed the rate of plasma refill, there where greater increases in serum Cr in those undergoing ultrafiltration with no improvement in any clinical parameter (weight loss, rehospitalization, death) [9]. In the double-blind Renal Optimization Strategies Evaluation (ROSE) trial, 360 subjects with ADHF and CKD were randomized in a factorial with 2:1 active treatment: placebo, such that approximately one third of the population received, in addition to standard diuretic-based decongestive therapy, low-dose dopamine (2 μg/kg/min infusion) while another third received low-dose nesiritide (human recombinant B-type natriuretic peptide, 0.005 μg/kg/min infusion for 72 h) [3]. Primary endpoints were total cumulative urine volume and change in serum cystatin-C. While the dopamine and nesiritide strategies both resulted in larger increases in urine output as compared to the placebo control (228 and 278 ml, respectively), these changes were small and not statistically significant. Cystatin C levels were not elevated (~1.1 mg/dl) at baseline, and there were no meaningful changes in this measure. There were no significant differences in symptoms, days alive and free from HF hospitalization at 60 days, or mortality. Of note, the rates of type 1 CRS were ~24 % in the ROSE trial and were unaffected by treatment allocation.
For both dopamine and nesiritide, there have been a significant number of trials and analyses with larger sample sizes showing no benefit in critically ill patients. For low-dose dopamine (<5 μg/kg/min), a meta-analysis of 3,359 subjects in 61 trials showed that, while dopamine resulted in a 24 % rise in urine output, there was no difference in death or the need for kidney replacement therapy [2]. Here, ROSE can add to treatment inferences in that dopamine resulted in a smaller increase in urine output (~3 %) and no impact on cystatin-C or hospitalization or death. For nesiritide, the ASCEND-HF (Acute Study of Clinical Effectiveness of Nesiritide and Decompensated Heart Failure) trial randomized 7,141 patients with ADHF to 24–168 (median 42 h) hours of nesiritide (2 μg/kg bolus then 0.01 mcg/kg/min) or placebo [9]. While nesiritide was associated with minor improvements in symptoms at 6 and 24 h, there were no differences in prespecified primary endpoints including rehospitalization and death. There were no differences in the rates of >25 % reduction in eGFR. Rates of AKI or type 1 CRS were again not reported. For nesiritide, ROSE adds the understanding that longer durations of a lower dose infusion with no bolus again confers no benefit over placebo.
Where did the NHLBI Heart Failure Trials network fail? Perhaps the lack of phenotyping played a role in failing to identify a population for benefit. Beyond hemodynamic characterization, blood and urine biomarkers should be used to understand the risk for and presence of both subclinical and clinical AKI [13]. The ROSE trial measured N-terminal pro B-type natriuretic peptide, but did not use it to phenotype patients into treatment subsets. In the future, the natriuretic peptides should be used to not only confirm ADHF but also to characterize patients along with other guidelines recommended tests, including galectin-3, troponin I or T, and ST2 [14]. Urine should be tested for tubular cell cycle arrest markers as well as markers of tubular injury, such as neutrophil gelatinase associated lipocalin, kidney injury molecule-1, interleukin-18, L-type fatty acid binding protein, and others [15]. It is possible that combination of these markers could identify ideal subsets for benefit or harm for particular clinical approaches.
A standard Kidney Disease International Global Outcomes (KDIGO) definition of AKI would have been more desirable as a formal primary endpoint in trials of type 1 CRS; since this definition of AKI in the setting of ADHF needs confirmation of translation into hard outcomes such has HF hospitalization and death [4]. Can we find an optimal treatment pathway using conventional therapies which are decades old in randomized trials of ADHF? The DOSE, CARRESS-HF, and ROSE trials suggest we cannot. Thus, future trials will need to target novel therapies and innovative ways (clinical exam, imaging, biomarkers) of identifying patients who are most likely to have the pathophysiology we are targeting with a new therapy.
Ularitide
With the failure of nesiritide to improve clinical outcomes in ADHF, is there a role for any form of natriuretic peptide in the prevention of type 1 CRS? If hemodynamic assessment is important for patient selection, then patients with intact perfusion but pulmonary congestion, possibly with ischemic cardiomyopathy could be an ideal subset for a novel natriuretic peptide which has natriuretic and diuretic properties on the kidneys as well as anti-ischemic and lusitropic properties on the myocardium. Ularitide is the chemically synthesized analogue of urodilatin, a human endogenous natriuretic peptide that regulates renal sodium and water excretion and is expressed in the kidney. After expression in the distal tubular cells, urodilatin is luminally secreted and binds mainly downstream in the inner medullary-collecting duct to specific natriuretic peptide receptors (NPR-A, NPR-B and also to other natriuretic peptide receptors), resulting in the activation of the intracellular guanylyl cyclase domain and generation of cyclic guanosine monophosphate (cGMP). The main pharmacological effects of exogenously administered ularitide are specific vasodilation (of renal, pulmonary, and coronary arteries, and peripheral arteries and veins), bronchodilation, diuresis and natriuresis, modulation of intrarenal blood flow, as well as inhibition of renal sodium reabsorption and of regulatory cardiac neurohormonal systems (sympathetic nervous system, renin-angiotensin system, endothelin).
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


