The aim of the present study was to report our additional experience with transcatheter closure of the patent ductus arteriosus in 65 consecutive patients using the new Amplatzer duct occluder. The median patient age was 3.6 years (range 0.2to 12), and the median weight was 10.5 kg (range 4 to 38). The device was a modified Amplatzer duct occluder made of fabric-free fine Nitinol wire net in to 2 very low profile disks with an articulated connecting waist. It is delivered through a 4Fr to 5Fr delivery sheath. The device was permanently implanted in 62 of 65 patients. The mean patent ductus arteriosus diameter (at the pulmonary end) was 3.6 ± 1.3 mm (range 0.5 to 5.5). The mean device diameter (waist diameter) was 4.2 ± 1.5 mm (range 3 to 6). Complete echocardiographic closure of the PDA at 1 month follow-up was observed in 61 (98%) of 62 patients. Immediately after the procedure, mild left pulmonary stenosis (peak pressure gradient of 8, 10, and 12 mm Hg) in 3 of 63 patients. Device embolization in 1 patient was the main complication of the procedure. No other complications were observed. In conclusion, catheter closure using the Amplatzer duct occluder II is an effective and safe therapy for most patients with patent ductus arteriosus. Additional studies are required to document its efficacy, safety, and long-term results in a larger patient population.
The design of previously used occluders for catheter closure of a patent ductus arteriosus (PDA) is not ideal for this purpose, and their use has been associated with several drawbacks, including device protrusion into the left pulmonary artery or the descending aorta, especially in small children with certain anatomic PDA types. Recently, Thanopoulos et al and Forsey et al reported quite satisfactory results with catheter closure of PDA using the Amplatzer duct occluder (ADO) II, a new device designed by the AGA Medical (Golden Valley, Minnesota). In the present study, we report our additional experience with transcatheter closure of the PDA in 65 consecutive patients using the ADO II.
Methods
A total of 65 consecutive patients from 3 cardiac centers in Greece underwent attempted PDA occlusion using the ADO II. The criteria for inclusion in the present study were a left-to-right shunt across the PDA, body weight ≥4 kg, and narrowest PDA diameter of ≤5.5 mm. The median patient age was 3.6 years (range 0.2 to 12), and the mean weight was 10.5 kg (range 4 to 38). Of the 65 patients, 22 were <12 months old (body weight 4 to 8 kg). Of these 22 patients, 11 had a body weight of ≤6 kg. Of the 65 patients, 38 had echocardiographic evidence of significant shunt through the PDA, with left atrial enlargement and ventricular volume overload. Patients with additional cardiac anomalies that would require cardiac surgery and aortopulmonary window-type PDAs were excluded from the present study. Informed parental consent was obtained for each patient.
The design of the new Amplatzer duct occluder (AGA Medical) has been described previously in detail. In brief, the ADO is made of fabric-free, fine Nitinol (NMT Medical, Boston, Massachusetts) wire net into 2 very low profile disks with an articulated connecting waist ( Figure 1 ). It is deployed through the pulmonary artery or the aorta.

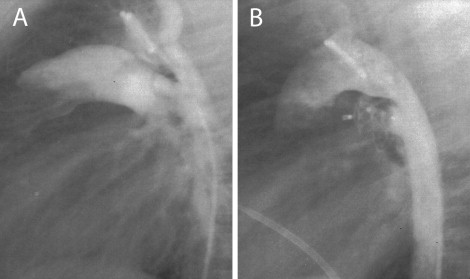
The technique of transcatheter closure of the PDA using the ADO II is similar to that described for the standard ADO ( Figure 2 ). The device size was selected according to the narrowest diameter of the ductus along its length. For a ductus <2.5 mm, an ADO II of 3 mm was used. In patients with larger ductal diameter (≤5.5 mm), a device of ≤1.5 mm larger than the ductus was used. According to the manufacturer’s instructions, a device 4 and 6 mm in length was used for short (<5 mm) and long (>5 mm) PDAs, respectively. Pull-back pulmonary and aortic pressure tracings and pulmonary arteriography and descending aortography were performed after device placement to document the presence of residual shunts and left pulmonary artery or aortic obstruction. Once the optimal position was confirmed, the devices were released by counterclockwise rotation of the delivery cable. Repeat aortography was performed to check for residual shunts. All patients were discharged 24 hours after the procedure with no medication. Chest radiography and complete 2-dimensional and color Doppler echocardiographic studies were performed on all patients at 24 hours and 1 month after the procedure.

The results are presented as the mean ± SD, with confidence intervals given, as applicable.
Results
According to the alphanumeric classification proposed by Krichenko et al, 37 patients had PDA type A, 10 had PDA type E, 5 PDA type B, 7 PDA type C, and 6 PDA type D. Delivery of the device was successful in all but 3 patients. In 5 patients with small PDAs (0.5 to 0.8 mm) and oblique pulmonary insertion, the ductus could not be crossed from the pulmonary artery. In these patients, the ADO II was successfully implanted using a femoral artery approach and a 4Fr delivery sheath. The mean PDA minimal diameter determined by aortography was 3.6 ± 1.3 mm (range 0.5 to 5.5). The mean device diameter was 4.2 ± 1.5 mm (range 3 to 6). In 3 patients (small infants 2 to 3 months old; body weight 4 to 5 kg) with a type A PDA measuring 4 to 4.5 mm in size and 6 to 7 mm in length, successive deployment of an ADO II 5 to 6 mm and 4 to 4 mm, respectively, resulted in significant left pulmonary artery and aortic obstruction. Thus, the occluder was repositioned within the sheath, and the ductus was closed successfully without complications using a 3 to 4 mm ADO II. In 2 small patients (body weight 4 and 5 kg) with a large 3.8 and 5 mm type C PDAs, respectively, deployment of the left disk of the ADO II caused significant aortic obstruction. Therefore, the ADO II was implanted within the ductus, resulting in complete closure without pulmonary or aortic obstruction ( Figure 3 ). In 2 small infants (2 and 3 months old, body weight 4 and 6 kg, respectively) with a large 5-mm, type A with shallow ampulla and type B PDA, respectively, successive deployment of a 4 to 4-mm and 3 to 4-mm occluder, respectively, resulted in significant pulmonary and aortic obstruction. Thus, the device was not released and was removed, and the patients were referred for surgical closure. Four infants with significant pulmonary hypertension (mean pulmonary artery pressure range 38 to 42 mm Hg) showed immediate improvement, with a reduction of the mean pulmonary artery pressure to 20, 25, 26, and 28 mm Hg. In 1 patient (4-month-old infant) with a large type A PDA, the device (owing to underestimation of the size of the ductus) embolized into the left pulmonary artery. All attempts to retrieve the occluder failed; therefore, it was removed surgically. Complete angiographic closure was observed in 59 (95%) of the 62 patients. In 3 patients, trivial residual shunt was present after the procedure. Immediately after the procedure, mild left pulmonary stenosis (peak pressure gradient of 8, 10, and 12 mm) was present in 3 (infants 2 and 3 months old) of the 62 patients. No obstruction of the left pulmonary artery or the descending aorta was noted in the remaining patients. Also, 6 temporary losses occurred of the arterial pulse and 2 minor (a groin hematoma and a minor bleeding) assorted procedural complications developed. All patients had good distal lower extremity pulses 24 hours after the procedure. No other complications were observed.