Fig. 6.1
Measurement of intraluminal PCO2 by tonometry. The tonometer is placed in the stomach. The mucosal PCO2 (PmCO2) and the gastric PCO2 (PgCO2) are equal because of the rapid diffusion of CO2 over the membrane layers. The balloon of the tonometer is also CO2 permeable; therefore, the PCO2 measured by the tonometer demonstrates the actual mucosal values (Adapted from Kolkman and Mensink [3], with permission)
Studies dating back to the early 1970s have used measurement of PCO2 in the mesenteric tract as an indicator of several pathophysiological processes and as a guide for treatment to improve the outcome of critically ill patients [16–19]. Measurement of PCO2 can easily be performed using mesenteric tonometry. The tonometer is a balloon-tipped catheter, which can be placed in the stomach, jejunum, or colon and is attached to a modified capnograph.
Tonometry has the potential to detect ischemia defined as insufficient delivery and/or consumption of oxygen for metabolic demands irrespective of flow and mesenteric perfusion. Initially, tonometry calculated the mesenteric mucosal pH derived from the PCO2 measured in the mesenteric lumen and the blood bicarbonate level using the Henderson-Hasselbalch equation [20]. However, this has major drawbacks as it is influenced by systemic variations in blood flow, which not necessarily reflects the true balance between oxygen supply and demand of the mesenteric tract [20, 21]. Therefore, it is replaced by the PCO2 gradient, defined as the difference between luminal and arterial PCO2. Since CO2 diffuses rapidly over the membrane layers, the mesenteric PCO2 in the lumen equals the PCO2 in the mucosa. Mucosal ischemia will invariably be associated with increased mesenteric PCO2 [3]. This is less influenced by systemic variation and is currently the preferred tonometric parameter for detection of ischemia.
Initially, the detection of mesenteric ischemia by tonometry was performed using meals as provocation [22]. However, this was not successful with an accuracy ranging from 0 to 100 % for ischemia as it was influenced by ongoing acid production of the stomach and direct influence of the meal on gastric PCO2 [23–25].
It was not until the development of the gastric exercise tonometry (GET) and the 24-hour tonometry by Kolkman et al. that tonometry could be used as a practical, validated diagnostic test for the detection of ischemia [4, 26, 27]. GET requires submaximal exercise, i.e., cycling, to trigger mesenteric ischemia. The concept is very similar to the commonly used exercise testing for assessment of cardiac ischemia. The prolonged, 24-hour combined gastric and jejunal tonometry requires standardized test meals as a provocation of mesenteric ischemia [5, 8].
Procedure
Gastric Exercise Tonometry
Gastric exercise tonometry (GET) testing is performed according to a standard protocol [1, 27]. Patients are not allowed to drink or eat prior to the procedure and acid suppression will be administered. The tonometer, a balloon-tipped nasogastric tube, is placed 60 cm from the tip of the nose assuming intragastric position. Gastric and arterial PCO2 measurements are performed before, during, and after the 10-min submaximal exercise. Gastric PCO2 measurements are measured by inflation of a semi-permeable balloon with saline and repeated aspiration of the gas content of the balloon. The tonometer is attached to a modified capnograph. A radial catheter is inserted to allow for arterial PCO2 measurements. To define a test result as pathologic, all three of the following criteria have to be met [4]:
1.
Gastric-arterial gradient >0.8 kPa after peak exercise
2.
Increase in gastric PCO2 from baseline to peak exercise
3.
Arterial lactate level <8 mmol/l
24-Hour Tonometry
This method consists of prolonged, 24-hour combined gastric and jejunal tonometry using standardized meals with a large metabolic demand as a provocation of mesenteric ischemia.
Tonometer catheters are inserted both in the stomach and jejunum by endoscopy and fluoroscopy. Gastric and jejunal PCO2 levels are registered every 10 min using a computer assisted data-collection program. Continuous intravenous administration of proton pump inhibitors and gastric pH measurements are performed to maximally control acid suppression (Fig. 6.2).
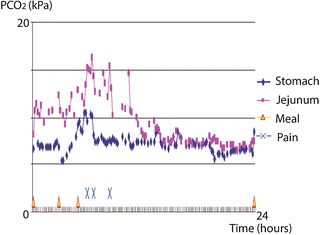

Fig. 6.2
Results of 24-hour tonometry in a patient with CMI. Abnormal postprandial increases in jejunal PCO2 values are seen with a close association with the onset of abdominal pain symptoms
Threshold values for elevated PCO2 levels are gastric or jejunal PCO2 > 12.0 kPa after breakfast or a bread meal, >13.6 kPa after dinner, or >10.6 kPa after ingestion of a compound solution. The criteria for a pathologic test result are [5, 28].
1.
Elevated PCO2 values after at least three meals or
2.
Combination of elevated PCO2 after at least one meal and a median PCO2 > 8.0 kPa measured between meals
Test Meals
Patients are required to consume standardized meals during the test [5]. To minimize the disturbing effects of meals on the intragastric PCO2 measurements explained by buffering and dilution, standardized meals are developed to optimally control the gastric and jejunal environment. The CO2-producing and CO2-absorbing capacities of these meals are minimal. Nevertheless, they maximally challenge the mesenteric arterial blood flow due to the large metabolic oxygen demand [29].
Meals consisting of a solution of carbohydrates, protein, and fat with a high caloric content and a low volume show the most significant increase in the blood flow of the mesenteric arteries [29, 30]. With this in mind, compound solution meals are the ideal meals showing maximum peak in PCO2 after ingestion. Consumption of carbonated liquids is prohibited during the test.
Diagnostic Value of Tonometry
Sensitivity and Specificity
In two large cohort studies including patients suspected of CMI, the diagnostic efficacy of GET was evaluated. With a sensitivity of 78–85 % and a specificity of 82–92 % for the detection of CMI, GET appears to be a reliable diagnostic tool in the assessment of CMI [4, 31]. The sensitivity and specificity of 24-hour tonometry for the detection of CMI are similar to GET with a sensitivity and specificity of 77–91 % and 94–100 %, respectively [5, 28]. The combination of clinical features with 24-hour tonometry has a sensitivity of 88 %. Adding radiological imaging by means of CTA or MRA, this can raise up to 90–91 % [28, 32].
Tonometry After Intervention
Repeated GET showed normalization or improvement in 88–100 % of patients with sustained relief of symptoms after treatment [1]. In patients with persistent symptoms, repeated GET showed improved in only 29 % of the patients, whereas in 71 % unchanged or worsened results were observed [1]. This indicates an accurate correlation between gastric mucosal ischemia and tonometry results.
Visible Light Spectroscopy
Background
Functional tests such as gastric exercise tonometry (GET) and 24-hour tonometry both seem to be accurate for detection of mesenteric ischemia [1, 5, 31, 32]. Unfortunately, the wider use of mesenteric tonometry is hampered by its cumbersome and invasive nature; therefore, the need for a better, more patient-friendly test remained. This led to the development of visible light spectroscopy (VLS). VLS, also known as reflectance spectrophotometry, enables direct measurement of the adequacy of mucosal perfusion [7]. It is a relatively new technique that noninvasively measures capillary hemoglobin oxygen saturation using white light delivered by a fiberoptic probe during endoscopy. The marked difference in the absorption spectra of oxygenated and deoxygenated hemoglobin makes direct measurement of the percent saturation of the mucosal hemoglobin possible. Using real-time signaling, artifacts as those caused by scattering can also be eliminated [7]. Connected to a device, continuous display of the mucosal oxygen saturation on a screen is possible [6, 7].
VLS has been used for evaluation of intensive care patients and assessment of anastomotic strength in esophageal and colorectal anastomoses and to determine microvascular perfusion during reconstructive surgery [33–35]. It can also increase endoscopic detection of mesenteric tumors [36].
Furthermore, VLS appears to be of great value as a new and less invasive diagnostic tool in patients suspected of CMI [6]. Since the measured oxygen saturation measurements reflect the adequacy of mucosal perfusion, events that decrease the delivery of oxygen to the mesenteric mucosa (i.e., mesenteric artery stenosis) will result in lower mucosal hemoglobin oxygen saturations [6]. VLS can easily be incorporated in diagnostic strategies as endoscopy is often performed early in the diagnostic work-up of these patients with abdominal pain.
Procedure
VLS is performed during upper endoscopy under conscious sedation [6, 7]. Patients are not allowed to drink or eat before the procedure. Butylscopalamine is admitted intravenously before the start of VLS measurements in order to prevent luminal spasms and optimize the readings. Peripheral oxygen saturation and heart rate are continuously monitored and to minimize the effect of confounding factors of concomitant cardiopulmonary diseases, peripheral saturation should be above 94 %. If necessary, oxygen (FiO2 21 %) can be administered.
The VLS measurements are performed using a fiberoptic catheter-based oximeter that can be passed through the endoscope. After irrigation of the target area to remove bile remnants, point measurements of the oxygen saturation are performed at three locations: antrum of the stomach, duodenal bulb, and descending duodenum. The probe is positioned approximately 1–5 mm above the mucosa (Fig. 6.3). Once a stable reading is obtained with less than 5 % variation in readout as seen on the display, the actual measurement can be performed. Three repeated readings will be taken of each location. The average of the three readings per location will be regarded as the most accurate reflection of mucosal oxygen saturation of that specific location.
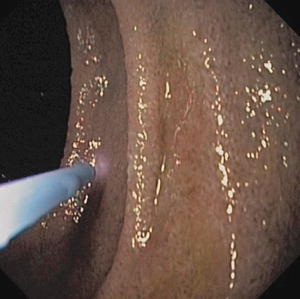

Fig. 6.3
Mucosal oxygen saturation using VLS. The probe is placed 1–5 mm directly above the mucosa of the stomach and duodenum
Based on the cutoff values determined in a large cohort study in CMI suspected patients, measurements are positive for ischemia when the measured saturation is:
<63 % in the antrum and/or
<62 % in the duodenal bulb and/or
<58 % in the descending duodenum [6]
Diagnostic Value of VLS
Sensitivity and Specificity
VLS is a validated diagnostic method to correctly detect CMI with a sensitivity and specificity of 90 % and 60 %, respectively [6]. The high sensitivity and low specificity are the consequence of the established cutoff values for each of the specific sites of the mesenteric tract as calculated by van Noord et al [6]. These cutoff values were based on a trainee data set of patients diagnosed with CMI using mesenteric tonometry and were additionally validated in a confirmation cohort.
With these cutoff values, the ability to distinguish patients with CMI from those without is the highest and no patients with CMI were missed, as earlier studies have shown that undiagnosed and untreated patients with ischemia have higher morbidity and mortality rates [10, 31]. However, the higher sensitivity results in a higher rate of false positives.
VLS After Intervention
After successful intervention, improved VLS measurements can be observed in patients with CMI [6]. Among the patients with relief of symptoms one year after intervention, 80 % showed improved or even normalization of the oxygen saturation measurements by VLS measurements. At the same time, in all patients with persistent symptoms after intervention, no improvement in the oxygen saturation measurements was observed [6]. Furthermore, selection for treatment based on standardized diagnostic work-up including radiological imaging and oxygen saturation measurements by VLS accommodates for a sustained response in 70 % of the patients with the clinical suspicion of CMI [37].
Clinical Considerations
Comparison Tonometry Versus VLS
Both VLS as tonometry have a high diagnostic accuracy for correctly diagnosing CMI [4, 5, 28, 31, 32]. In the assessment of CMI, tonometry allows the clinician to differentiate patients with asymptomatic single- or multivessel stenosis of the mesenteric arteries and patients in whom the abdominal symptoms are induced by mesenteric ischemia. The newly developed VLS has also shown its value and even appears to be slightly better in identifying patients with CMI [4–6, 28, 31].
Limitations of Tonometry
GET requires submaximal exercise to trigger mesenteric ischemia. However, many patients in this category are not able to perform suboptimal exercise for sufficient period of time to assess CMI due to age or concomitant disease [16]. Also, despite frequent monitoring of serum lactate as a measure for exercise intensity, the level of exercise in patients is still difficult to control.
As the name indicates, 24-hour tonometry requires 24-h hospitalization. This is inconvenient and expensive. Furthermore, suboptimal gastric acid suppression and meal-related CO2 production may affect the tonometry results leading to false-positive outcomes therefore requiring the use of continuous gastric acid suppression and standardized meals [39].
Limitations of VLS
The measures of performance of VLS are based on data from only one large cohort study and has not yet been conducted or reproduced by other research groups [6]. This study compared mucosal oxygen saturation measurements in patients with CMI with a control group of non-CMI patients. However, the latter consisted of patients with a clinical suspicion of CMI, which is not the ideal. A control group consisting of healthy volunteers is needed to avoid selection bias and to obtain normal values for mucosal oxygen saturation measurements in order to optimize the cutoff values.
With the current cutoff values and the high sensitivity of 90 %, no patient with CMI should be missed. However, the mean of mucosal saturation measurements in patients with CMI shows great variation and the range of measurements is large. Due to small changes in the position of the probe during the measurements, small variations in the oxygen saturation can occur with the possibility of not acquiring the actual mucosal oxygen saturation. Especially if the obtained value is around the specified cutoff value, it can be difficult to classify the measurement as either positive or negative for mucosal ischemia. Therefore, the average of three repeated measurements of each location is regarded as the most accurate reflection of the mucosal oxygen saturation of that location [6].
Another limitation of mucosal oxygen measurements using VLS is that the current technique causes restrictions regarding the place and time of the measurements. Mucosal ischemia might be patchy and because VLS is limited to point measurements could therefore be missed with VLS [6, 7]. Furthermore, there is a possibility that mucosal ischemia only occurs postprandially, in response to an increased metabolic demand, or is exercise related indicating a time-dependent relation. VLS measurements are performed in a fasting state during endoscopy. In theory, patients with CMI with less impaired mesenteric blood flow could show low-normal or even normal mucosal saturation measurements in this fasting situation and can therefore be missed using VLS.
Conclusion
The choice for the functional test depends on the patient’s condition, presence of comorbidity, and clinical symptoms. For instance, patients with exercise-induced symptoms are far better detected with GET rather than with 24-hour tonometry or VLS [38, 39]. For patients with postprandial complaints, 24-hour tonometry or VLS would be the most suiting technique. Furthermore, the experience of the treating physician with the technique and equipment is important.
VLS is minimally invasive and can be performed in almost all patients [6, 8]. Patients with a clinical suspicion of CMI have to undergo endoscopy early in the work-up, during which measurements with VLS can be easily performed. It only requires an additional two minutes during endoscopy which, in contrast to 24-hour tonometry, can be performed in the outpatient clinic. Therefore, it is also less expensive.
The diagnostic value of tonometry for the detection of CMI is more accurate than VLS. Reproducibility of tonometry has been shown in numerous studies, whereas these validations are lacking for VLS [4, 32, 37]. VLS is a promising diagnostic test for detection of CMI and shows excellent correlation with tonometry; however the established cutoff values need to be validated [37]. In conclusion, the strength of evidence for accurately detecting CMI is greater for tonometry than for VLS and is therefore the preferred method.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


