The objective of the present study was to investigate the relation of ventricular premature complex (VPC) burden, origin, and electrocardiographic characteristics with left ventricular function and survival. Of 1,589 study patients, 388 (25%), 610 (38%), and 591 (37%) had low (<1,000/24 hours), moderate (1,000 to 10,000/24 hours), and high (>10,000/24 hours) VPC burden, respectively. Twenty-three percent of study patients had a left ventricular (LV) ejection fraction <50% (8% in low-, 20% in moderate-, and 36% in high-VPC-burden groups, p <0.001). High VPC burden was associated with lower LV ejection fraction in the presence (r = −0.17, p <0.001) and absence (r = −0.20, p <0.001) of heart diseases. The Kaplan-Meier survival estimates showed a significant difference among the 3 VPC burden groups (p = 0.046). The survival rates were significantly lower for patients with a VPC coupling interval of ≥480 ms than those with a VPC coupling interval of <480 ms (p = 0.002) and lower for those with a VPC QRS duration of ≥150 ms than those with a VPC QRS duration of <150 ms (p <0.001). In conclusion, high VPC burden is detrimental to LV systolic function. Broader VPC QRS duration and longer VPC coupling interval adversely impact on long-term survival.
Highlights
- •
To investigate the relation of ventricular premature complex (VPC) burden, origin, and electrocardiographic characteristics with left ventricular function and survival.
- •
Of 1,589 study patients, 388, 610, and 591 had low (<1,000/24 hours), moderate (1,000 to 10,000/24 hours), and high (>10,000/24 hours) VPC burden, respectively.
- •
The high VPC burden was associated with lower left ventricular ejection fraction in the presence (r = −0.17, p <0.001) and absence (r = −0.20, p <0.001) of heart diseases.
- •
The Kaplan-Meier survival estimates showed a significant difference among the 3 VPC burden groups (p = 0.046).
- •
The survival rates were significantly lower for patients with VPC coupling interval of ≥480 ms than the counterpart group of <480 ms (p = 0.002) and lower for those with VPC QRS duration of ≥150 ms than the group of <150 ms (p <0.001).
- •
High VPC burden is detrimental to left ventricular systolic function. Broader VPC QRS duration and longer VPC coupling interval adversely impact on long-term survival.
Ventricular premature complex (VPC) is well recognized as a dysrhythmic manifestation of structural heart diseases. Yet, VPC has been considered a benign rhythm in the absence of structural heart disease. Not until the past decade has the concept of VPC-induced cardiomyopathy become a subject of great interest. The causal relation of VPC and cardiomyopathy was initially proposed by Duffee et al in 1998 when pharmacologic suppression of VPC in patients with presumed idiopathic dilated cardiomyopathy (DCM) subsequently improved left ventricular (LV) systolic dysfunction. Since then, other series of observational studies have showed similar findings. The objectives of the present study were to investigate (1) the relation of VPC burden with LV function and survival in a large patient cohort with presence or absence of heart diseases and (2) the effects of VPC origin, QRS duration, and coupling interval on LV ejection fraction (LVEF) and long-term survival.
Methods
The eligible study cohort was identified through the Rochester Medical Index database. The Rochester Medical Index classified 5,183 patients’ diagnoses of VPC from 2005 to 2010 using an internal coding system based on the Hospital International Classification of Diseases Adapted , eighth revision. Patients were then matched with the Current Procedural Terminology billing code for Holter monitors. The indications for Holter were mainly for investigating symptoms or arrhythmic risk stratification. Only the Holter reports within 6 months of VPC diagnosis were used for analysis. Burden of VPC was presented as the ratio of total VPC to total heart beats per 24 hours. They were classified into 3 groups in accordance with daily VPC burden (<1,000/24 hours, 1,000 to 10,000/24 hours, and >10,000/24 hours). The study was approved by the Mayo Clinic Institutional Review Board.
Patient medical histories were reviewed thoroughly by 2 electrophysiologists (Y-HL and LZ). The clinical symptoms associated with the diagnosis of VPC were collected from the electronic medical record. Patients were identified as having the presence of heart diseases if they had a clinical diagnosis of coronary artery disease (angiographically confirmed >75% stenosis in ≥1 coronary arteries), DCM, hypertrophic cardiomyopathy, and valvular heart disease.
All available 12-lead electrocardiograms and 12-lead Holter monitors at the time of VPC diagnosis were reviewed. The morphology of VPC was classified if it is monomorphic or a predominant pattern on the electrocardiogram and 12-lead Holter (if it is available). The following QRS features were used to define the site of origin: (1) LV outflow tract (OT): morphologic characteristics of right bundle branch block with inferior axis, tall R waves in the inferior leads, and QS complexes in aVR and aVL leads or characteristics of left bundle branch block but with a more prominent R wave amplitude and duration in lead V 1 and early transition in V 2 or V 3 ; (2) right ventricular (RV) OT: morphologic characteristics of left bundle branch block with inferior axis, tall R waves in the inferior leads, QS complexes in aVR and aVL, or a QS or small R wave in lead V 1 with R transition in V 3 and V 4 ; (3) LV non-OT: morphologic characteristics of right bundle branch block in the absence of features of LV OT; and (4) RV non-OT: morphologic characteristics of left bundle branch block without the typical inferior axis or features of RV OT.
All patients had echocardiographic reports within 6 months of VPC diagnosis. Echocardiographic parameters included LVEF (derived from 2-dimensional measurement or M-mode), LV end-diastolic dimension, and LV end-systolic dimension. Survival status as of January 14, 2013, for the entire cohort, including patients who did not return for clinical follow-up, was obtained from an institutionally approved national death and location database.
Results are presented as mean (SD) or number of patients (percentage). Two-sample t tests were used for comparison of continuous variables among the groups; chi-square tests were used for comparison of categorical variables. Survival was calculated with the Kaplan-Meier method. The multivariate model was determined with all clinical variables of statistical significance in the univariate models (p <0.05).
Results
The study cohort contained 1,589 patients with a diagnosis of VPC (male gender 55%, mean [SD] age 61 years [16]). Of these patients, 388 (25%), 610 (38%), and 591 (37%) had VPCs <1,000/24 hours (low VPC burden), 1,000 to 10,000/24 hours (moderate VPC burden), and >10,000/24 hours (high VPC burden; Table 1 ).
| Variable | Number of VPCs/24 Hours | p Value | ||
|---|---|---|---|---|
| <1,000 (n = 388) | 1,000–10,000 (n = 610) | >10,000 (n = 591) | ||
| Age, mean (SD), (years) | 59.8 (16) | 59.9 (16.9) | 62.7 (16.3) | .004 |
| Male | 183 (47%) | 321 (53%) | 375 (63%) | <.001 |
| Hypertension | 117 (30%) | 175 (29%) | 189 (32%) | .46 |
| Diabetes mellitus | 20 (5%) | 40 (7%) | 52 (9%) | .08 |
| Heart disease | 71 (18%) | 188 (31%) | 251 (42%) | <.001 |
| Coronary artery disease | 41 (11%) | 69 (11%) | 75 (13%) | .57 |
| Dilated cardiomyopathy | 21 (5%) | 98 (16%) | 158 (27%) | <.001 |
| Hypertrophic cardiomyopathy | 5 (1%) | 4 (1%) | 1 (0.2%) | .10 |
| Valvular disease | 1 (0.3%) | 6 (1%) | 4 (1%) | .40 |
| Congenital heart disease | 6 (2%) | 11 (2%) | 6 (1%) | .51 |
| Arrhythmogenic right ventricular dysplasia | 0 (0%) | 2 (0.3%) | 1 (0.2%) | .50 |
| Atrial fibrillation | 57 (15%) | 66 (11%) | 57 (10%) | .04 |
| Sleep apnea | 17 (4%) | 25 (4%) | 20 (3%) | .70 |
| Dyspnea | 54 (14%) | 86 (14%) | 101 (17%) | .26 |
| Dizziness | 37 (10%) | 31 (5%) | 41 (7%) | .03 |
| Palpitations | 120 (31%) | 163 (27%) | 101 (17%) | <.001 |
| Chest discomfort | 33 (9%) | 65 (11%) | 40 (7%) | .06 |
| Syncope | 20 (5%) | 35 (6%) | 23 (4%) | .32 |
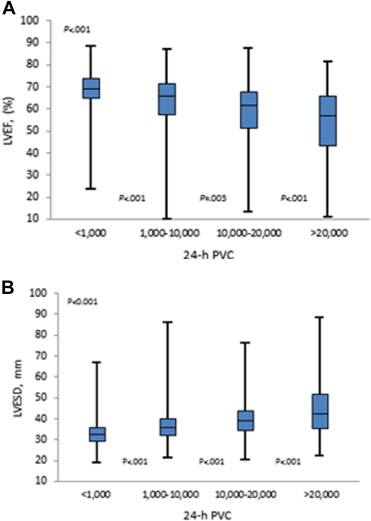
| LVEF, mean (SD), (%) | 61.8 (8.4) | 56.6 (11.9) | 51.2 (13.3) | <.001 |
| LVEF <50% | 31 (8%) | 122 (20%) | 213 (36%) | <.001 |
| LVEDD, mean (SD), (mm) | 48.5 (5.7) | 51.1 (6.3) | 54.2 (7.4) | <.001 |
| LVESD, mean (SD), (mm) | 31.1 (5.9) | 34.6 (7.7) | 39.1 (9.5) | <.001 |
| NSVT, mean (SD), (runs) | 0.2 (0.9) | 9.6 (63.4) | 184.2 (616.5) | <.001 |
| Longest run, mean (SD), (beats) | 0.4 (1.7) | 3.1 (27.6) | 55.1 (580.2) | .02 |
| Heart rate, mean (SD), (bpm) | 71.8 (12.7) | 71.9 (10.3) | 76.1 (9.9) | <.001 |
| VPC origin | <.001 | |||
| Right ventricular outflow tract | 25 (6%) | 75 (12%) | 143 (24%) | |
| Right ventricular non-outflow tract | 62 (16%) | 118 (19%) | 82 (14%) | |
| Left ventricular outflow tract | 11 (3%) | 53 (9%) | 94 (16%) | |
| Left ventricular non-outflow tract | 81 (21%) | 206 (34%) | 197 (33%) | |
| Multiple morphology | 7 (2%) | 23 (4%) | 41 (7%) | |
| Undefined | 202 (52%) | 135 (22%) | 34 (6%) | |
| VPC coupling interval (ms) | 505 (121) | 495 (98) | 492 (89) | 0.14 |
| VPC duration (ms) | 136 (18) | 141 (20) | 143 (22) | <.001 |
| β-Blocker | 123 (32%) | 214 (35%) | 207 (35%) | .48 |
| Calcium channel blocker | 25 (6%) | 22 (4%) | 25 (4%) | .10 |
| Antiarrhythmic drugs | 40 (10%) | 28 (5%) | 24 (4%) | <.001 |
| Mexiletine | 2 (1%) | 1 (0.2%) | 1 (0.2%) | .49 |
| Flecainide | 9 (2%) | 6 (1%) | 1 (0.2%) | .004 |
| Sotalol | 8 (2%) | 7 (1%) | 9 (2%) | .51 |
| Amiodarone | 12 (3%) | 12 (2%) | 10 (2%) | .31 |
| Propafenone | 10 (3%) | 2 (0.3%) | 4 (1%) | .001 |
| ACEI/ARB | 70 (18%) | 143 (23%) | 192 (32%) | <.001 |
LVEF tended to be lower and LV dimension larger as VPC burden increased (all p <0.001). The VPC QRS was more widened as the VPC burden increased (p <0.001). Of the study cohort, 1,079 patients (68%) had no documented heart diseases. VPCs originating from the RV were more common in the absence of heart diseases (34% vs 27%, p = 0.008); conversely, VPCs from the LV were more frequently seen in patients with heart diseases (50% vs 36%, p <0.001).
To determine the effect of VPC burden on LV systolic function, the group with high VPC burden was further broken down to VPC levels of 10,000 to 20,000/24 hours and >20,000/24 hours. The LVEF tended to be lower as the VPC burden increased ( Figure 1 ). Conversely, the LV end-systolic diameter became larger ( Figure 1 ). Table 2 shows a significant correlation of VPC frequency with LVEF and LV end-systolic diameter regardless of presence or absence of heart diseases (all p <0.001). The incidence of LVEF <50% was 8%, 20%, and 36% in the groups with low, moderate, and high VPC burden, respectively (p <0.001).
| Relation of VPC Frequency | Presence of Heart Diseases | Absence of Heart Diseases | ||
|---|---|---|---|---|
| Correlation ∗ | p Value | Correlation ∗ | p Value | |
| With left ventricular ejection fraction | −0.18 | <.001 | −0.20 | <.001 |
| With nonsustained ventricular tachycardia | 0.54 | <.001 | 0.42 | <.001 |
| With left ventricular end-diastolic dimension | 0.24 | <.001 | 0.12 | .001 |
| With left ventricular end-systolic dimension | 0.24 | <.001 | 0.18 | <.001 |
Although the VPC burden from OT areas tended to be greater than that from non-OT areas ( Table 3 ), more patients had VPCs originating from non-OT locations than from OT locations (70% vs 30%). The VPC burden was similar between RV and LV, yet LVEF was lower when VPCs originated from the LV than from the RV (p = 0.001).
| Groups | Number of VPCs/24 Hours | LVEF |
|---|---|---|
| Outflow tract (N = 365) | 19095 (14979) | 54.3 (12.4%) |
| Non-outflow tract (N = 836) | 12778 (13749) | 53.8 (13.1%) |
| p value | <.001 | 0.50 |
| Right ventricular (N = 418) | 15297 (13903) | 55.4 (12.2%) |
| Left ventricular (N = 550) | 15986 (14744) | 52.7 (13.1%) |
| p value | 0.46 | 0.001 |
| Polymorphic (N = 64) | 19272 (18298) | 46.5 (14.0%) |
| Monomorphic (N = 968) | 15689 (14384) | 53.9 (12.8%) |
| p value | 0.06 | <.001 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


