Previous studies have suggested that angiographically detected peristent contrast staining (PSS) at follow-up may predict subsequent very late stent thrombosis. The Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial was a dual-arm, factorial, randomized trial in patients with ST-segment elevation myocardial infarctions. All follow-up angiograms (1,330 lesions in 1,115 patients, median time 13.3 months) without major cardiovascular events before follow-up angiography were analyzed at a core laboratory blinded to clinical events for the presence of PSS (defined as contrast staining outside the stent contour extending to ≥20% of the stent diameter). Corresponding follow-up intravascular ultrasound (IVUS) data (275 lesions in 248 patients) were also evaluated to assess the mechanisms of PSS. PSS was present in 23 patients (2.1%) at follow-up and was not more common with paclitaxel-eluting than with bare-metal stents. All 6 PSS patients with follow-up IVUS had stent malapposition (vs 41.2% malapposition in the follow-up IVUS cohort). Comparing poststent and follow-up IVUS, 2 patients had late acquired and 4 had persistent malapposition; all 6 showed positive vessel remodeling from baseline to follow-up (mean vessel area 22.0 ± 8.0 to 32.4 ± 11.7 mm 2 , p = 0.07). During 3-year follow-up, stent thrombosis developed in no patient with PSS compared with 8 PSS-negative patients (0% vs 0.8%, p = 0.68). The rates of revascularization and major adverse cardiovascular events were also not increased in PSS patients. In conclusion, in the large-scale HORIZONS-AMI trial, PSS at angiographic follow-up was infrequent and was associated with late stent malapposition and positive remodeling but was independent of stent type. Identification of PSS was not associated with subsequent stent thrombosis.
Although drug-eluting stents (DES) have reduced restenosis, the occurrence of very late stent thrombosis remains a major concern, in particular with first-generation DES. One risk factor for very late stent thrombosis is late stent malapposition, which occurs more commonly with DES than bare-metal stents and more commonly after stenting in the setting of acute myocardial infarction compared with stable angina. However, the detection of late stent malapposition requires intracoronary imaging. Recently, Imai et al reported that angiographic peristent contrast staining (PSS)—contrast outside the stent contour extending ≥20% of the stent diameter—is a potential surrogate for late stent malapposition and predicts very late stent thrombosis. In the present study, we assessed baseline and 13-month follow-up angiographic and intravascular ultrasound (IVUS) data and 3-year clinical follow-up data from the Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial to identify the frequency and ability of PSS to predict very late stent thrombosis in patients treated with DES or bare-metal stents in the setting of acute myocardial infarction.
Methods
HORIZONS-AMI was a prospective, open-label, multicenter, dual-arm, 2 × 2 factorial, randomized trial in patients with ST-segment elevation myocardial infarctions (STEMIs) presenting <12 hours after symptom onset who underwent primary percutaneous coronary intervention. The 2 randomization arms consisted of (1) the direct thrombin inhibitor bivalirudin alone versus heparin plus a glycoprotein IIb/IIIa inhibitor (1:1 randomization), and (2) Taxus paclitaxel-eluting stents (PES) versus otherwise equivalent Express bare-metal stents (3:1 randomization; both Boston Scientific Corporation, Natick, Massachusetts).
IVUS substudy sites were preselected on the basis of their agreement to perform baseline (poststent) and 13-month follow-up IVUS on consecutive patients in the angiographic follow-up cohort until ≥300 consecutive IVUS cases were enrolled. Because clinical outcomes after follow-up angiographic detection of PSS in native coronary arteries were the focus of the present study, we excluded patients who had major adverse events such as target lesion revascularization, myocardial infarctions, stroke, and stent thrombosis before angiographic and IVUS follow-up as well as lesions in saphenous vein grafts.
Coronary angiograms at baseline, immediately after the intervention, and at follow-up were analyzed at the Angiographic Core Laboratory of the Cardiovascular Research Foundation (New York, New York) using the CMS-GFT algorithm (MEDIS Medical Imaging Systems, Leiden, the Netherlands). Qualitative and quantitative analyses were done using standard published methods. Flow was graded according to the Thrombolysis In Myocardial Infarction (TIMI) trial classification. PSS was defined as contrast staining outside the stent contour extending ≥20% of the stent diameter as measured on quantitative coronary angiography.
IVUS was performed after successful, uncomplicated stent implantation with motorized pullback at 0.5 mm/s to include the stent and >5-mm segments proximal and distal to the stent. Allowable IVUS systems included iLab, Galaxy, ClearView (all with Atlantis SR Pro, 40-MHz catheters; Boston Scientific Corporation, Fremont, California), and In Vision Gold with 20-MHz EagleEye catheters (Volcano Corporation, Rancho Cordova, California). All IVUS studies were archived onto S-VHS tape or digital media and sent to the IVUS core laboratory (Cardiovascular Research Foundation) for off-line quantitative and qualitative analyses by individuals blinded to treatment allocation. Quantitative analysis was performed with validated planimetry software (echoPlaque; INDEC Systems, Inc., Mountain View, California). IVUS measurements were performed millimeter by millimeter beginning 5 mm distal to the distal stent edge and continuing through the stent to a point 5 mm proximal to the proximal stent edge, including the external elastic membrane, stent, lumen, neointimal hyperplasia (stent minus lumen), and malapposition cross-sectional areas. Stent malapposition, synonymous with incomplete stent apposition, was defined as blood speckle behind stent struts not overlying a side branch.
Patients returned for angiographic and IVUS studies at 13 months after the index procedure. Clinical follow-up was performed at 30 days, 1 year, 2 years, and 3 years. Major adverse cardiovascular events included cardiac death, myocardial infarction, and ischemia-driven target vessel revascularization. Safety major adverse cardiovascular events included death, myocardial infarction, stroke, and stent thrombosis. Myocardial infarction was defined as the electrocardiographic appearance of pathologic Q waves in ≥2 contiguous leads associated with an elevated creatine kinase-MB fraction or an elevated creatine kinase-MB fraction >2 times the upper limit of normal in the absence of pathologic Q waves. Stent thrombosis was defined according to the Academic Research Consortium definitions.
Statistical analysis was performed using SAS version 9.2 (SAS Institute Inc., Cary, North Carolina). Categorical variables were compared using chi-square or Fisher’s exact tests and are presented as frequencies, and continuous variables were compared using Mann-Whitney U tests and are presented as median (interquartile range [IQR]). The cumulative incidence of stent thrombosis and safety major adverse cardiovascular events after the diagnosis of PSS was estimated using Kaplan-Meier plots and compared using the log-rank test. Hazard ratios and 95% confidence intervals were calculated. Receiver-operating characteristic curve analysis was used to identify the cut-off value of maximum malapposition area best separating angiographically positive versus negative PSS. A p value <0.05 was considered statistically significant.
Results
Baseline patient characteristics are listed in Table 1 . PSS was present in 23 of 1,115 patients (2.1%) at angiographic follow-up. The rates of bivalirudin versus heparin plus a glycoprotein IIb/IIIa inhibitor and PES versus bare-metal stent use were not statistically different in patients with versus without PSS. Patients with PSS had a higher rate of previous coronary bypass graft surgery and a higher rate of thienopyridine use at 3 years compared with those without PSS.
| Variable | PSS | No PSS | p Value |
|---|---|---|---|
| Bivalirudin | 65% (15/23) | 51% (553/1,092) | 0.17 |
| PES | 78% (18/23) | 76% (826/1,092) | 0.77 |
| Age (yrs) | 62.4 (53.2–70.0) | 59.2 (51.9–67.9) | 0.46 |
| Men | 65% (15/23) | 79% (860/1,092) | 0.13 |
| Hypertension ∗ | 48% (11/23) | 52% (566/1,092) | 0.70 |
| Hyperlipidemia † | 61% (14/23) | 42% (454/1,092) | 0.06 |
| Diabetes mellitus | 13% (3/23) | 15% (163/1,092) | 1.00 |
| Current smokers | 48% (11/23) | 48% (525/1,092) | 0.98 |
| Renal insufficiency ‡ | 4% (1/23) | 1% (15/1,092) | 0.29 |
| Previous myocardial infarction | 4% (1/23) | 2% (25/1,092) | 0.42 |
| Previous percutaneous coronary intervention | 9% (2/23) | 7% (81/1,092) | 0.69 |
| Previous coronary bypass | 9% (2/23) | 1% (15/1,092) | 0.05 |
| Symptom onset–to–balloon time (hours) | 3.6 (2.4–5.3) | 3.7 (2.7–5.5) | 0.61 |
| Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers at discharge | 70% (16/23) | 86% (941/1,092) | 0.03 |
| Thienopyridine at 1 yr | 78% (18/23) | 75% (8,091/1,080) | 0.71 |
| Thienopyridine at 3 yrs | 50% (11/22) | 27% (283/1,053) | 0.016 |
| Aspirin at 3 yrs | 96% (21/22) | 97% (1,019/1,051) | 0.50 |
∗ Defined per patient report or medical chart review documentation of history of systemic arterial hypertension or current diagnosis.
† Defined per patient report or medical chart review documentation of history of hyperlipidemia or current diagnosis.
‡ Defined as baseline creatinine clearance <60 ml/min calculated using the Cockcroft-Gault formula.
As listed in Table 2 , lesions with PSS were more frequently located in the right coronary artery than those without PSS (p = 0.04). Lesion length was longer (p = 0.02) and reference vessel diameter was larger in lesions with PSS versus lesions without PSS (p = 0.06). PSS lesions were associated with an increased frequency of angiographic (1) aneurysm and ectasia at baseline and follow-up, (2) calcification at baseline, and (3) postprocedural dissection and thrombus. On follow-up angiography, reference vessel diameter was significantly greater in patients with versus without PSS (p = 0.01), consistent with positive remodeling.
| Variable | PSS | No PSS | p Value |
|---|---|---|---|
| Baseline findings | |||
| Target coronary artery | |||
| Left anterior descending | 24% (5/21) | 40% (516/1,304) | 0.14 |
| Right | 67% (14/21) | 44% (576/1,304) | 0.04 |
| Left circumflex | 10% (2/21) | 16% (212/1,304) | 0.56 |
| Lesion length (mm) | 20.9 (12.9–24.1) | 15.0 (10.4–20.4) | 0.02 |
| Reference vessel diameter (mm) | 2.96 (2.58–3.66) | 2.88 (2.55–3.21) | 0.064 |
| Minimal luminal diameter (mm) | 0.00 (0.00–0.50) | 0.18 (0.00–0.65) | 0.49 |
| Diameter stenosis (%) | 100 (79–100) | 94 (76–100) | 0.51 |
| TIMI flow grade 0 or 1 | 62% (13/21) | 57% (658/1,158) | 0.6 |
| Thrombus | 86% (18/21) | 69% (903/1,303) | 0.10 |
| Calcification | 62% (13/21) | 33% (430/1,302) | 0.005 |
| Aneurysm | 10% (2/21) | 1% (14/1,304) | 0.03 |
| Ectasia | 15% (3/20) | 3% (39/1,304) | 0.02 |
| American Heart Association/American College of Cardiology type B2/C | 100% (21/21) | 85% (1,108/1,304) | 0.06 |
| Postprocedural findings | |||
| Stent length (mm) | 31.5 (24.6–44.5) | 20.6 (16.0–27.9) | <0.0001 |
| In-lesion minimal luminal diameter (mm) | 2.65 (2.13–2.88) | 2.37 (2.04–2.70) | 0.10 |
| In-lesion diameter stenosis (%) | 19 (14–23) | 18 (13–25) | 0.95 |
| Thrombus | 10% (2/21) | 1% (14/1,305) | 0.03 |
| Dissection | 10% (2/21) | 0% (6/1,305) | 0.006 |
| TIMI flow grade 3 | 86% (18/21) | 91% (1,050/1,159) | 0.44 |
| Follow-up findings | |||
| Reference vessel diameter (mm) | 3.09 (2.83–3.47) | 2.87 (2.55–3.21) | 0.01 |
| In-lesion minimal luminal diameter (mm) | 2.38 (1.92–2.72) | 2.13 (1.73–2.46) | 0.06 |
| In0lesion diameter stenosis (%) | 23 (17–32) | 25 (17–36) | 0.42 |
| Binary restenosis | 9% (2/23) | 8% (104/1,275) | 0.71 |
| Ulceration | 17% (4/23) | 0.2% (3/1,307) | <0.0001 |
| Aneurysm | 17% (4/23) | 0.3% (4/1,307) | <0.0001 |
| Ectasia | 13% (3/23) | 0.6% (8/1,307) | 0.0007 |
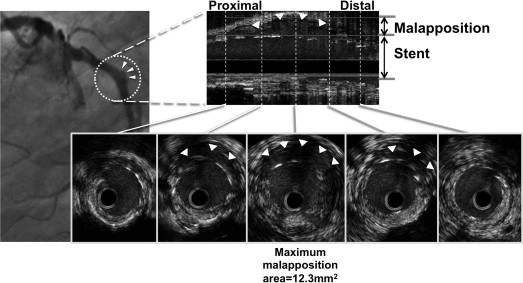
In 275 lesions with follow-up IVUS, malapposition was observed in 115 (41.8%). Among these 115 lesions, PSS was observed by angiography in 6 lesions (6 patients), whereas 109 lesions had late stent malapposition without angiographic PSS. Thus, the sensitivity of PSS to predict IVUS malapposition was 5.2% (6 of 115), and the specificity was 100% (160 of 160). For these 6 PSS lesions, the median IVUS maximum malapposition area was 6.5 mm 2 (IQR 5.3 to 11.1). Figure 1 shows an example of a lesion with PSS observed by IVUS. Among 6 lesions with PSS and late stent malapposition, 2 had late acquired malapposition, and 4 had persistent malapposition (malapposition present at baseline and persisting at follow-up). The maximum malapposition site showed positive vessel remodeling in all 6 lesions from baseline to follow-up; the average external elastic membrane area increased from 22.0 ± 8.0 to 32.4 ± 11.7 mm 2 (p = 0.07). Among lesions with late stent malapposition, percentage net volume obstruction ([neointimal volume/stent volume] × 100) was not statistically different in lesions with versus without PSS (median 1.0% [IQR 0.4% to 6.7%] vs 4.2% [IQR 1.5% to 9.5%], p = 0.32). Receiver-operating characteristic curve analysis identified a follow-up IVUS maximum malapposition area >2.7 mm 2 (area under the curve 0.97) as best separating PSS-positive from PSS-negative lesions.