Movement of Water across the Capillary Wall
The capillary wall (here taken to include the wall of postcapillary venules) is very permeable to water. However, although individual water molecules can move freely between the plasma and the tissue spaces, the net flow of water across the capillary wall is very small. This flow is determined by a balance between two forces or pressures that are exerted across the wall of the capillaries. These are hydrostatic pressure, which tends to drive water out of the capillary, and colloid osmotic pressure, which tends to draw water into capillaries from the surrounding tissue spaces. The sum of these two pressures at each point along the capillary is equal to a net pressure that will be directed either out of or into the capillary, and the net flow of water is proportional to this net pressure. The classic Starling equation describes the relationship between net flow (Jv) and the hydrostatic and osmotic pressures:
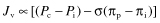
The hydrostatic force (Pc − Pi) is equal to the difference between the blood pressure inside the capillary (Pc) and the pressure in the interstitium around the capillary (Pi). Pc in blood-perfused capillaries ranges from about 35 mmHg at the arteriolar end of the capillaries to about 15 mmHg in the venules. Pi is slightly subatmospheric in many tissues (−5 to 0 mmHg), due to a suction of fluid from the interstitium by the lymphatic capillaries. The greater pressure inside the capillary tends to drive filtration, the movement of water out into the tissues.
As described in Chapter 20, the capillary wall acts as a semipermeable membrane or barrier to free diffusion, across which electrolytes and small molecules pass with much greater ease than plasma proteins. A substance dissolved on one side of a semipermeable membrane exerts an osmotic pressure that draws water across the membrane from the other side. This osmotic pressure is proportional to the concentration of the substance in solution, and is also a function of its permeability. Substances that can easily permeate a barrier (in this case the capillary wall) exert little osmotic pressure across it, whereas those that permeate less readily exert a larger osmotic pressure. For this reason, the osmotic force across the capillary wall is largely a result of the relatively impermeant plasma proteins, in particular albumin. The osmotic pressure exerted by plasma proteins is referred to as the colloid osmotic or oncotic pressure.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


