Fig. 34.1
Structure of human saphenous vein. (Left) Whole transverse section of non-distended human saphenous vein (SV) where the intima, surrounding the lumen (L) is thrown into folds. (Right) High magnification of the wall of the SV showing the three distinct layers (I intima, M media, A adventitia). The arrows indicate the vasa vasorum
1.
The innermost layer, the intima, consisting of little more than a thin basement membrane and its endothelial lining.
2.
The middle layer, the media, consisting mainly of vascular smooth muscle cells (VSMCs) separated by collagen in which the vasa vasorum is located.
3.
The outermost layer, the adventitia, is the thickest layer and is composed of collagen fibers and fibroblasts that merge with the surrounding connective tissue and fat. Also, within the adventitia are embedded the vascular (autonomic) nerves and vasa vasorum. Surrounding the adventitia is a pronounced cushion of perivascular fat (PVF).
The inner border of the media and intima is divided by the internal elastic lamina. In medium-sized veins, such as the SV, the intima is thrown into folds, and there may be signs of thickening due to the presence of smooth muscle cells and collagen within regions of neointimal hyperplasia. These features are only seen in ‘normal,’ non-distended/immersion-fixed sections and often not represented by published examples that have been perfusion fixed at ∼100 mmHg compared with the vein’s normal ∼10 mmHg basal pressure. The media and adventitia are divided by the external elastic lamina, which is less distinct in veins than arteries. The vascular nerves are located in the adventitia, and also within this layer is a microvessel network, the vasa vasorum, which penetrates the media and may extend into the vessel lumen. During conventional vein harvesting, all vessel layers are damaged to varying degrees.
Intima and Endothelium
The single layer of cells lining the vein lumen, the endothelium, is affected during harvesting. There may be some ‘mechanical’ detachment of endothelial cells caused during removal and handling during surgery. The most striking effect is the dramatic endothelial denudation caused by distension at pressures of up to or over 700 mmHg [6]. While these effects are apparent by light microscopy [7], further shape changes and cell detachment are revealed using electron microscopy [8, 9] (Fig. 34.2). Apart from the striking effects observed on the endothelium, distension-induced ‘smoothing’ of the intima has been described where the folds that are evident in non-distended vein segments are absent in veins that have been subjected to high-pressure intraluminal distension [10] (Fig. 34.3). There may also be evidence of damage to cells within the intima as well as rupture of the internal elastic lamina.
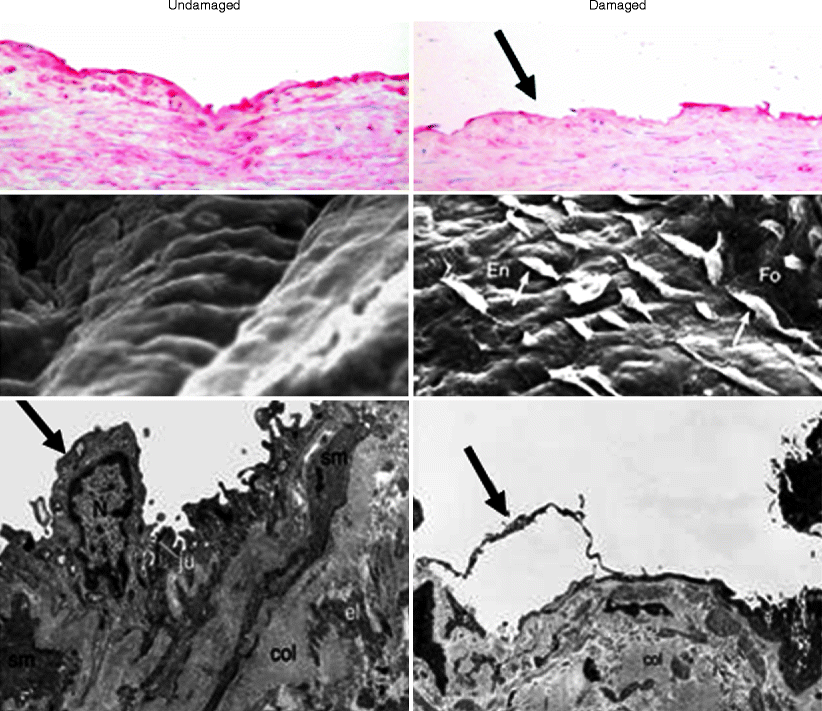
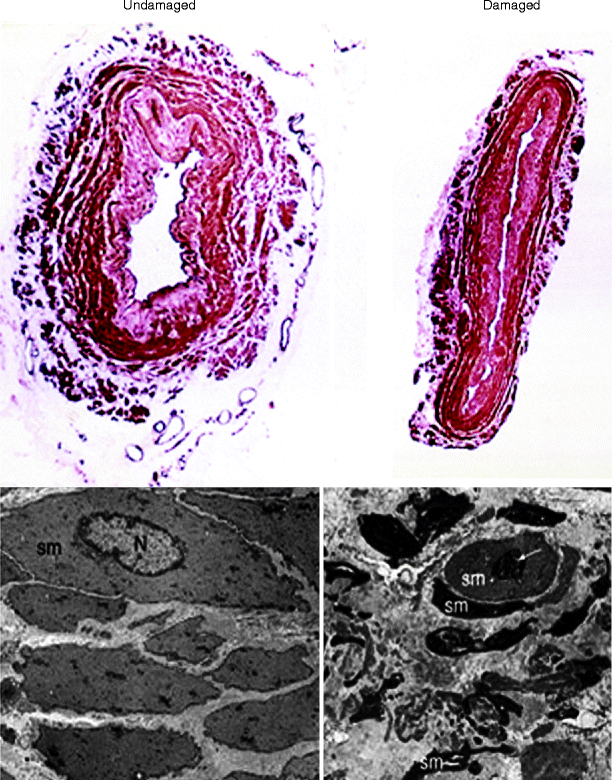

Fig. 34.2
Endothelium of undamaged and damaged human saphenous vein. The panels on the left are representative examples from undamaged (no-touch) veins and on the right are damaged (conventional) veins. The top panels show immunohistochemical localization of luminal endothelium (identified using CD31: red stain), which is continuous in the undamaged vein. In the damaged (conventional) vein, there are large areas of endothelial denudation (arrow). The middle panels show examples of scanning electron micrographs of en face preparations of human SV where there is a smooth, intact endothelium lining of undamaged veins but denudation and shape change of endothelial cells of the damaged vein. The lower panels show examples of transmission electron micrographs of luminal endothelial cells. There is a marked alteration in endothelial cells (En, arrows) in damaged versus undamaged veins. sm smooth muscle, col collagen, N nucleus, Fo luminal fold, ju junction, el elastin (Modified from Refs. [8, 9])

Fig. 34.3
Media and vascular smooth muscle of undamaged and damaged human saphenous vein. (Top panels) Representative transverse sections of undamaged vein (left) and damaged (right) human SV. (Lower panels) Transmission electron micrographs where the undamaged vascular smooth muscle cells are uniform in shape in the undamaged vein but exhibit dramatic shape changes in the damaged cells. sm smooth muscle, N nucleus, small white arrow nuclear division (Modified from Ref. [8])
Media
While the VSMCs that predominate within the media control venous tone, they may also alter their phenotype from contractile to synthetic. In this state these cells migrate and proliferate and are associated with the medial and intimal thickening observed in occluded vein grafts. Although there may be some damage to the media caused by manipulation by surgical instruments, the most pronounced effect is due to high-pressure distension during harvesting. This additional insult causes considerable thinning of the vessel wall [10] (Fig. 34.3), presumably because of overstretching of smooth muscle cells exposed to high/raised circumferential pressures. Again, dramatic cellular changes have been reported within the media, with striking shape changes to VSMCs as well as intracellular effects, including increased signs of nuclear division [8]. In addition to the effects on medial VSMCs, there are signs of damage to the vasa vasorum, where many of these microvessels appear collapsed and their endothelial cells misshapen and/or occluded by erythrocytes [8].
Adventitia
It is the adventitia, the outermost layer of the SV, that is generally removed or severely damaged during conventional harvesting but spared when using Souza’s ‘no-touch’ technique [11] (Figs. 34.3 and 34.4). First, along with its adjacent connective tissue and cushion of surrounding fat, the adventitia constitutes a robust perivascular structure that protects the vein against the effects of altered hemodynamics once implanted into the coronary arterial system. In addition to this mechanical role, the adventitia contains the major proportion of autonomic nerves innervating the vessel, as well the vasa vasorum, microvessels responsible for the exchange of gases and nutrient supply to the vessel wall. Although the excised vein is essentially denervated, removal of the adventitia will further reduce the perivascular nerves, although these have been shown to proliferate in experimental porcine grafts [12, 13].
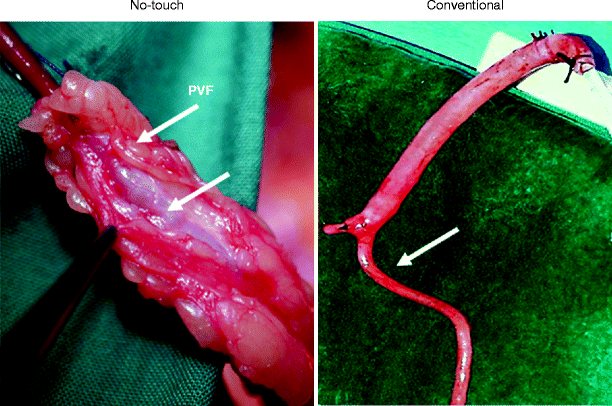

Fig. 34.4
No-touch and conventional saphenous vein used as bypass grafts in CABG. (Left) The no-touch vein is removed complete with its cushion of surrounding perivascular fat (PVF). Handling the vein via this cushion prevents the vein from going into spasm (lower arrow). (Right) Conventionally harvested vein is stripped of surrounding tissue causing spasm (arrow) that is overcome using high-pressure saline distension (shown at upper segment of the vein). To the left is a tied off side branch
Perhaps the most serious consequence of removing the adventitia is the damage caused to the vasa vasorum (Fig. 34.5). There is convincing evidence from experimental animal models that occlusion of the vasa vasorum by a close-fitting external collar [14] or adventitial removal [15] leads to neointimal hyperplasia and atherosclerosis, both features associated with vein graft failure. It has been suggested that these effects are mainly due to ischemia of the vessel’s media, and this is supported by studies showing that reduced transmural oxygen levels are detected in occluded femoral arteries in an experimental model of atherosclerosis [14]. Interestingly, it has been shown that the vasa vasorum is affected by application of both dilator and constrictor compounds, suggesting that they play a role in the regulation of the tone of the vasa vasorum, implicating this microvessel network in conduit vessel physiology [16].
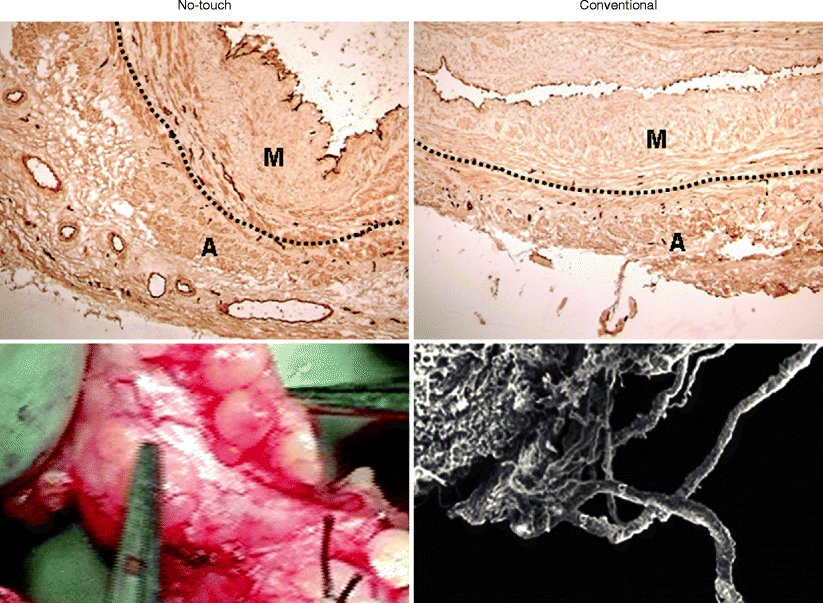

Fig. 34.5
Vasa vasorum of no-touch and conventionally harvested saphenous vein. Left panels show representative examples of a no-touch vein with adventitia intact. The lower panel shows retrograde blood flow through the adventitial vasa vasorum on release of vascular clamps at completion of graft insertion – evidence of luminal termination. Right panels show examples of conventionally harvested veins with much of the adventitia removed (top panel) with the lower panel showing a scanning electron micrograph of the damage to the adventitial vasa vasorum. The endothelial cells lining both the lumen and vasa vasorum in the top panels are identified using CD31 (dark immunostaining). The dotted line shows the external elastic lamina that separates the media (M) and adventitia (A)
Perivascular Fat
Using the no-touch harvesting technique, the SV is removed with minimal surgical damage and complete with its pronounced cushion of perivascular fat (PVF) (Fig. 34.4). In this way, the vein retains its normal architecture, providing superior graft patency when compared with veins prepared by conventional methods. When performing conventional harvesting, the vein is stripped of much of its surrounding tissue, a procedure that inflicts considerable vascular damage. In addition, a high proportion of veins to go into spasm, which is overcome using high-pressure intraluminal distension. We have evidence that the intact PVF of SVs harvested by the no-touch technique plays an essential role in its success. Since the vein is handled during surgery by the PVF, direct contact by surgical instruments and consequent spasm are avoided, distension is unnecessary, and the luminal endothelium remains intact. In addition, the vasa vasorum is preserved and the supply of oxygen and nutrients to the graft wall maintained. The PVF also provides mechanical support to the vein once implanted into the coronary arterial circulation where it acts as a buffer, protecting the graft against arterial hemodynamics as well as preventing kinking of excessively long grafts (Fig. 34.6). Finally, the surrounding cushion of fat is a source of adipocyte-derived relaxing factors (ADRFs) [17–19], many of which are vasculoprotective. For example, PVF surrounding no-touch-harvested SVs obtained from patients undergoing CABG is a potential source of NO, a vasorelaxant factor with antithrombotic, anti-inflammatory, and antiproliferative properties [20, 21]. We have also identified leptin in extracts of PVF from human SV [22]. Positive immunostaining for leptin is associated with adipocytes of the PVF surrounding the SV, and, as an adipokine with both vasorelaxant and angiogenic properties, we hypothesize that this, and other adipocyte-derived factors such as adiponectin [23], may play an important role in the improved performance of no-touch SV grafts.
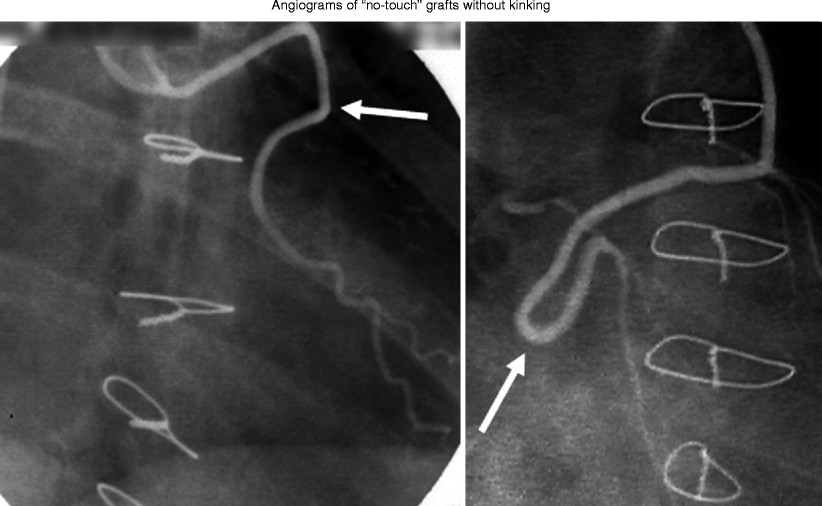

Fig. 34.6
Angiographic examples of no-touch saphenous vein grafts. Two examples of angiograms of no-touch saphenous vein grafts in CABG patients where the surrounding cushion of tissue prevents kinking (arrows), maintaining blood flow in excessively long grafts
Conventional Saphenous Vein Harvesting Technique
The SV has been the vessel of choice for autologous vein grafts since its introduction for CABG by Favarolo in 1969 [24]. The use of autologous grafts eliminates problems of tissue rejection and the need for tissue typing and matching. The SV also has a number of practical advantages: it is expendable, since lower limb drainage can rely solely on the deep venous system; its long length allows its use for multiple grafts, and its superficial position renders it easily accessible, facilitating its exposure at harvest [25]. Since its introduction as a graft, the SV has become the most commonly used conduit in patients undergoing CABG. However, the patency rate of this vessel is poor, with 15–25 % grafts occluding within 1 year and over 50 % patients requiring redo surgery within 10 years [26].
Although there may be minor modifications made by cardiac surgeons, during conventional harvesting, the SV is either harvested endoscopically or exposed by a longitudinal leg incision, where the surrounding connective tissue, including the adventitia, is stripped off and its side branches ligated. Generally, veins are then distended with saline to check for leakage, often at high pressure to overcome the spasm that occurs because of surgical trauma [25]. Rather than preserving their normal architecture, SV grafts are frequently prepared in such a way that they merely serve as channels (hence the general term “conduit”) for redirecting blood flow past occluded regions of the coronary vasculature in order to maintain or restore myocardial perfusion. For example, the original paper describing the use of the SV as a bypass graft for CABG states that, “Care must be taken to dissect only the vein, avoiding as much as possible the adventitia that surrounds it” [24]. These instructions have been taken by many cardiac surgeons to indicate that the vein should be stripped of surrounding tissue at harvesting. Consequently, a high proportion of SVs go into spasm, with the high pressure saline distension employed causing further damage to the vein [22, 25]. The potential effect of vascular damage on graft patency has been recognized for some time, and various atraumatic dissecting techniques have been introduced in an attempt to improve vein graft performance [11, 27, 28].
No-Touch Saphenous Vein Harvesting
The particular ‘no-touch’ technique that is the focus of this chapter was described by Souza in1996 [11]. Various minor modifications have been made subsequently, with details of the technique, including video footage, available online (http://mmcts.ctsnetjournals.org/cgi/content/full/2009/0731/mmcts.2008.003624) [29]. Briefly, the day before the operation, a Duplex examination of the SV should be performed in order to mark the course of the vein on the skin. This allows an incision to be made precisely over the vein to avoid creating a flap of subcutaneous tissue, thus reducing the risk of complications such as hematomas, seromas, or infection. Also, on the day of CABG, the SV is located and harvested with minimal delay. It is imperative to follow the surgical technique in all details from harvesting to implantation. A longitudinal incision is made over the skin and the required length of vein exposed, keeping its perivascular tissue in place (Fig. 34.4). After exposure, a margin of about 0.5 cm is created around the vein to include the fat pedicle using electrocautery, and all visible side braches are ligated and divided at the same level (0.5 cm from the vein). The SV, together with its cushion of surrounding tissue, is then separated from its bed using scissors and electrocautery. The vein should be left in situ and covered with a moistened compress at least until a few minutes after heparinization. This allows continuous heparinized blood perfusion to be carried out and obviates the need for rinsing or flushing the vein with saline solution. After removal, the vein is stored in heparinized blood obtained from the aortic cannula. While performing the anastomosis, the vein is handled via the surrounding cushion, thereby avoiding direct contact between the vein and instruments. This prevents spasm from occurring. After each completed distal anastomosis, the vein graft is briefly connected to the arterial cannula of the cardiopulmonary bypass system using a three-way stopcock to check for any leakage from the anastomosis or side branches. Manual distension using a syringe should be avoided, as this procedure damages the endothelial lining of the lumen [3, 7, 10]. After removal of the aortic cross-clamp, and before suturing the proximal anastomoses, the grafts are once again connected to the arterial line. This procedure assists in determining the graft length and maintains the vein in a dilated condition. Accordingly, this is a true “no-touch” technique as the SV is not handled with instruments nor is it distended or flushed during the whole procedure. SVs prepared by this no-touch technique exhibit an improved early graft performance [30, 31] with patency at 18 months for no-touch SV grafts of 95 % versus 89 % for conventional grafts and similar to the ITA. A long-term (mean 8.5 years) follow-up study described a no-touch SV graft patency that was comparable to the ITA (both 90 %) and superior to conventional SV grafts (76 %) [32].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


