Fig. 16.1
Critical aortic stenosis in a 26 weeks’ gestational age hydropic fetus. (a) Pre-intervention echocardiographic assessment. Color flow mapping across the aortic valve (indicated by an arrow) showing tiny forward flow (blue color). The red color displays the flow into the right ventricle across the tricuspid valve. The LV is conspicuously dilated and dysfunctional. The fetus is in severe heart failure resulting in hydrops. Asterisks show ascites. (b) The guidewire is clearly seen in the ascending aorta, which proves the aortic valve (indicated by an arrow) was crossed successfully. (c) The balloon (black asterisks) is inflated across the aortic valve. There is some pericardial effusion (white asterisks) that required drainage after dilation. (d) Immediate results after aortic valvuloplasty. There is significant improvement in antegrade flow across the aortic valve (indicated by an arrow) as shown by color flow mapping (blue color depicts a much wider vena contrata across the aortic valve). Abbreviations: LA left atrium, LV left ventricle, AAo ascending aorta, GW guidewire
2.
HLHS and intact or highly restrictive IAS [1–3]: A prenatal echocardiographic diagnosis of HLHS with either an intact IAS or a tiny (≤1 mm) atrial septal defect (ASD) or patent foramen ovale (PFO) and prominent flow reversal in the pulmonary veins should be made. Fetal atrial septostomy and ASD creation are ideally performed between 29 and 32 weeks’ gestation in order to endure until term (Fig. 16.2).
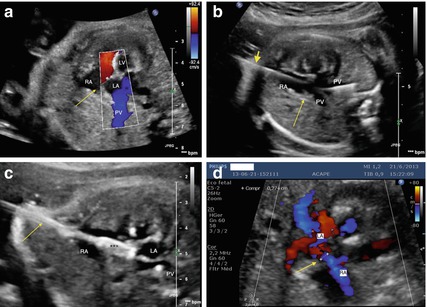

Fig. 16.2
Fetal atrial septostomy in a 29 weeks’ gestational age fetus with established hypoplastic left heart syndrome (a). Pre-intervention echocardiographic assessment. The LA is conspicuously dilated and the interatrial septum (indicated by an arrow) is almost intact. There is flow reversal to the pulmonary veins by color flow mapping (blue color). The red color displays the flow into the right ventricle across the tricuspid valve. The LV is hypoplastic and diffuse endocardial fibroelastosis can be seen as bright hyperechogenic areas. (b) The interatrial septum (indicated by a long and narrow arrow) was traversed with the Chiba needle (indicated by a short and broad arrow) and the guidewire is seen in a pulmonary vein. (c) A 4 × 10-mm coronary balloon (marked with black asterisks) is inflated across the interatrial septum up to the burst pressure reaching 4.7 mm in diameter. Note that the Chiba needle (indicated by an arrow) is perpendicular to the plane of the interatrial septum. (d) Post-intervention echocardiographic assessment on the following day after fetal atrial septostomy. A 2.8-mm atrial septal defect was created within the atrial septum (indicated by an arrow). Abbreviations: LA left atrium, RA right atrium, PV pulmonary vein, LV left ventricle
3.
PA/IVS or CPS/IVS and evolving HRHS [1–3]: Patients should have a prenatal echocardiographic diagnosis of PA/IVS or CPS/IVS with the following features: membranous pulmonary atresia, with identifiable pulmonary valve (PV) leaflets or membrane, no or minimal systolic opening, and no or minimal color Doppler ultrasound flow across the pulmonary valve (PV); an intact ventricular septum; left-to-right shunting across a patent ductus arteriosus (PDA); and right heart hypoplasia, with a tricuspid valve (TV) annulus Z-score below ≤ 2 and an identifiable but qualitatively small right ventricle (RV) with no evidence of RV growth after 2–4 weeks of serial echocardiographic evaluation. Cases with fetal diagnosis of major coronary-to-RV fistulas should be excluded. Pulmonary valvuloplasty is performed between 24 and 30 weeks’ gestation (Fig. 16.3).
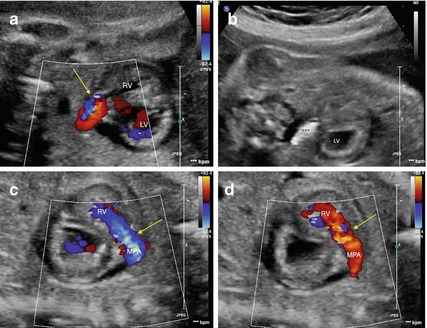

Fig. 16.3
Critical pulmonary valve stenosis in a 28 weeks’ gestational age fetus. (a) Pre-intervention echocardiographic assessment. Color flow mapping across the pulmonary valve (indicated by an arrow) shows tiny forward flow (in blue color). The red color depicts the retrograde flow across the ductus. The pulmonary valve annulus measured 4.0 mm. (b) A 4 × 10-mm coronary balloon (marked with black asterisks) is inflated across the pulmonary valve up to the burst pressure reaching 4.7 mm in diameter. (c) Immediate results after pulmonary valve dilation. Color flow mapping across the pulmonary valve (indicated by an arrow) shows significant improvement in forward flow across the valve (in blue color). (d) Color flow mapping across the pulmonary valve (indicated by an arrow) shows significant pulmonary insufficiency (in red color), which is a marker of effective dilatation. Abbreviations: RV right ventricle, LV left ventricle, MPA main pulmonary artery
4.
Critical AS, massive mitral regurgitation (MR), giant left atrium (LA), and hydrops [1, 2]: These fetuses have normal-sized LV and reversed flow in the TAA. Aortic valvuloplasty and atrial septostomy should be considered between 30 and 34 weeks’ gestation as a “salvage” procedure to diminish the risk of fetal loss due to conspicuous hydrops associated with pulmonary veins and right ventricular compression.
16.3 Pre-procedural Imaging and Planning
Fetal cardiac interventions should be performed by a multidisciplinary team. The fetal cardiologist is responsible for patient selection and pre- and post-procedural echocardiographic assessment. The fetal medicine specialist conducts fetal positioning and anesthesia and simultaneously controls the puncture needle and the ultrasound probe. The interventionalists (usually two) handle the catheters and wires while the fetal medicine specialist holds onto the needle to keep its position during the procedure.
16.4 Technique (Step-by-Step), Materials, and Tips and Tricks
We perform such interventions under maternal conscious sedation and regional spinal blockade conducted by an anesthesiologist [1, 2]. An appropriate fetal lie is achieved by external version. Maternal positioning is kept with left uterine displacement. To promote uterine relaxation mothers are given nifedipine 20 mg TID for 48–72 h, starting 12–24 h before the procedure. An occasional large polyhydramnios is evacuated using a 15-cm-long 21-G Chiba needle (Cook Inc, Bloomington, IN, USA). If ideal fetal positioning cannot be attained by external manipulation, the procedure should be abandoned. We do not perform any interventions through a maternal abdominal wall incision and uterus exposure. After optimal fetal position is achieved, the fetus is anesthetized using a mixture of fentanyl (5–10 μg/kg), pancuronium (10–20 μg/kg), and atropine (20 μg/kg) given intramuscularly or in the umbilical cord using a 21–22-G Chiba needle [1, 2].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


