We evaluated the feasibility and clinical utility of transesophageal echocardiography (TEE) in the early management of ischemic stroke. TEE was performed in consecutive patients with acute cerebral ischemia within 48 hours of symptoms onset. The data were analyzed by age (<55 vs ≥55 years), and the baseline stroke etiology was classified (determined vs undetermined). TEE was feasible in 660 (61%) of 1,080 patients. Left atrial abnormalities and complicated aortic plaques prevailed in older patients (p <0.05), irrespective of the stroke etiology. A patent foramen ovale prevailed in younger patients (p <0.05) but even in older patients was present in 13% of the determined and 31% of the undetermined stroke subgroups. Overall, high-risk and potentially high-risk cardioembolic sources were detected in 47% of the patients, and stroke etiology was consequently reviewed: 40% of the baseline undetermined strokes were reclassified as cardioembolic, and 29% of lacunar, 42% of large artery, and 30% of other determined-cause strokes were reclassified as concurrent etiology. Subsequently, according to the current guidelines, 12% of patients were reassigned from antiplatelet to anticoagulant therapy and 17% of patients were treated with high-dose statins; overall, secondary prevention treatment was modified in 26% of patients. In conclusion, TEE was feasible in about 2/3 of the patients investigated within 48 hours of the index event, contributed to stroke classification in 1/3 of cases, and guided secondary prevention therapy in 1/4 of patients. Therefore, TEE is useful for defining patients’ risk profile for stroke recurrence.
Cardioembolic strokes are reported to represent ≤25% of all strokes when identified at hospital entry according to the clinical features, electrocardiographic findings, and transthoracic echocardiographic findings. When embolic sources are more accurately researched using transesophageal echocardiography (TEE), their prevalence can reach 40%. However, limited consensus has been reached regarding its use in the acute phase of ischemic stroke, even though the early diagnosis of cardioembolic events could identify patients at greater risk of recurrence and death. The aim of the present study is to evaluate whether TEE performed within 48 hours of the ischemic stroke onset could help to refine the subtype classification and guide early tailoring of secondary prevention therapies.
Methods
From June 2004 to September 2007, all consecutive patients with ischemic stroke admitted within 24 hours from symptom onset to our Emergency Department Stroke Unit were enrolled in the study. At admission, the severity of the neurologic deficit was quantified using the National Institutes of Health Stroke Scale, and a score of <3 identified minor strokes. Transient ischemic attacks were defined according to the standard World Health Organization definition of the resolution of symptoms and signs within 24 hours, irrespective of the imaging results. Information on routine laboratory analyses (including complete lipid and coagulation profiles), cardiovascular risk factors (defined according to the published criteria ), and medical history were recoded at baseline. The presence of coronary heart disease was defined on the basis of a history of angina, previous myocardial infarction, previous coronary artery revascularization, or angiographically documented coronary lesions. Valvular heart disease was considered only if more than mild. All patients underwent plain brain computed tomography (and/or magnetic resonance imaging, when clinically indicated according to the guidelines ), duplex ultrasonography of the extracranial and intracranial arteries, and 12-lead electrocardiography. Venous ultrasound assessment of the lower limbs was performed in the case of clinical suspicion of deep vein thrombosis, according to the guidelines. The stroke subtypes were classified at baseline according to a modified version of the “Trial of Org 10172 in Acute Stroke treatment” (TOAST) criteria into 6 subgroups: large artery atherosclerosis, cardioembolism, small-vessel occlusion, other determined etiology, undetermined etiology, and concurrent etiology.
Within 24 hours of admission, all patients were scheduled for transthoracic echocardiography and TEE using a dedicated service of ultrasound cardiovascular imaging. The examinations were performed using a Philips Sonos 5500 Ultrasound System (Andover, Massachusetts) equipped with a 2.5- to 3.5-MHz scanning frequency, phased array transducer for transthoracic echocardiography and a 5.0-MHz multiplane transducer for TEE. Transthoracic echocardiography was performed before TEE in each patient, standard imaging planes were viewed, and the analysis performed according to the published guidelines. A right-to-left shunt using saline contrast injection was not assessed during the transthoracic echocardiographic study. TEE was performed according to a common protocol (after the patient had received local anesthesia and mild sedation), with particular emphasis on the potential embolic findings. One or more injections of intravenous sterile isotonic solution, before and after the patient performed a Valsalva maneuver, were routinely given to each patient to detect a right-to-left shunt. The Valsalva maneuver was considered correctly performed when the atrial septal membrane bulged into the left atrium. A patent foramen ovale (PFO) was diagnosed when >3 microbubbles were visualized in the left atrium within 5 cardiac cycles after complete opacification of the right atrium. Atrial septal aneurysm (ASA) was diagnosed when excessive membrane expansion was observed (>10 mm of bulging with a base span of ≥15 mm). Spontaneous echocardiographic contrast was characterized by slowly swirling intracavitary densities imaged with gain settings adjusted to distinguish the background noise, and it was noted only when continuously present at the preset gain (dense spontaneous echocardiographic contrast). Complicated aortic atheroma was defined as an aortic plaque ≥4 mm in thickness and/or ulcerated and/or with thrombus attached to the surface. No complications occurred during the procedures. All the studies were stored and subsequently reviewed by 2 independent observers (FP, EDA) and, in the event of discrepancies, by a third (SDC). Complicated aortic atheroma, left ventricular thrombi, left atrial and/or left atrial appendage (LAA) thrombi, and thrombogenic milieu (spontaneous echocardiographic contrast and/or LAA velocities <30 cm/s) were classified as high-risk cardiac sources of embolism. Uncomplicated aortic plaques, PFO with the Valsalva maneuver, and ASA (without PFO) were considered as potential risk sources. PFO at rest and PFO plus ASA were classified as potentially high-risk sources. After TEE, the TOAST classification was revised, such that patients presenting with high-risk and potentially high-risk sources were reclassified as “cardioembolic,” if no other plausible cause of stroke before TEE had been detected (i.e., classified as undetermined before TEE) and as “concurrent,” if a plausible cause of stroke had been detected before TEE (i.e., large artery, lacunar, or other determined causes before TEE). Subsequently, according to the transesophageal echocardiographic findings, patient treatment was revised, and, where necessary, the therapy was adjusted by the physicians according to the current guidelines.
The results are expressed as the absolute frequencies and relative percentages for categorical variables and as the mean ± SD or median and interquartile range for continuous variables. The baseline characteristics and transesophageal echocardiographic findings were compared according to age (≥55 vs <55 years) and gender using a t test for continuous variables and chi-square test for categorical variables. Similar analyses were used when comparing patients with determined (i.e., large artery, cardioembolic, lacunar, and other determined and concurrent causes) and undetermined causes of stroke. Statistical analysis was performed using Stata software, release 10 (StataCorp, College Station, Texas). A p value of ≤0.05 was considered statistically significant.
The institutional medical ethics committee approved the study, and all patients or appropriate relative, in the case of patient incapacity, provided written informed consent before enrollment.
Results
During the study period, 1,080 consecutive patients with ischemic stroke were admitted to our stroke unit within 24 hours of symptoms onset and were scheduled for TEE within 24 hours of admission. Of these, 420 patients (38.9%) could not undergo TEE. Of the 420 patients, 54 (5%) had an altered level of consciousness, 216 (20%) had severe dysphagia/dysphasia, 108 (10%) had died before completing the diagnostic course, 11 (1%) had refused to undergo TEE, and 30 (3%) had a priori major contraindications for anticoagulant therapy. Of the 420 patients, ½ were men, their mean ± SD age was 71.3 ± 12.9 years, and the median National Institutes of Health Stroke Scale on admission was 9 (interquartile range 5 to 16). According to the modified TOAST classification, 108 cases (25.7%) were classified as large artery atherosclerosis, 119 (28.3%) as cardioembolic, 87 (20.7%) as lacunar, 17 (4.1%) as other determined, 41 (9.8%) as concurrent etiology, and 48 cases (11.4%) as undetermined.
Thus, 660 patients underwent TEE. The baseline clinical and echocardiographic characteristics of the 660 patients are listed in Table 1 . As expected, the older patients had a greater prevalence of cardiovascular risk factors (e.g., atrial fibrillation, hypercholesterolemia, diabetes, and hypertension); however, no differences were observed between the men and women. In contrast, significantly more of the younger patients were current smokers. According to the modified TOAST classification at baseline, 403 strokes (61.0%) were classified as undetermined in the overall population. Of these, 300 (58.8%) were among patients aged ≥55 years and 103 (68.7%) among patients aged <55 years ( Figure 1 ).
| Variable | Overall | Age (yrs) | p Value | |
|---|---|---|---|---|
| <55 | ≥55 | |||
| Patients (n) | 660 | 150 (22.7%) | 510 (77.3%) | |
| Baseline clinical characteristics | ||||
| Men | 394 (59.7%) | 80 (53.3%) | 314 (61.6%) | 0.071 |
| Age (years) | 64.4 (13.5) | 44.7 (8.0) | 70.2 (8.2) | <0.001 |
| Symptoms onset to hospitalization <12 hours | 418 (63.3%) | 98 (65.3%) | 320 (62.8%) | 0.563 |
| Atrial fibrillation ⁎ | 76 (11.5%) | 5 (3.3%) | 71 (13.9%) | <0.001 |
| Previous transient ischemic attack | 107 (16.2%) | 15 (10%) | 92 (18.0%) | 0.019 |
| Previous stroke | 195 (29.5%) | 26 (17.3%) | 169 (33.1%) | <0.001 |
| Previous coronary heart disease | 76 (11.5%) | 10 (6.7%) | 66 (12.9%) | 0.034 |
| Valvular heart disease | 24 (3.6%) | 2 (1.3%) | 22 (4.3%) | 0.087 |
| Hypercholesterolemia | 180 (27.4%) | 24 (16.0%) | 156 (30.8%) | <0.001 |
| Diabetes | 120 (18.2%) | 11 (7.3%) | 109 (21.4%) | <0.001 |
| Systemic arterial hypertension | 410 (62.4%) | 50 (33.8%) | 360 (70.7%) | <0.001 |
| Smoking status | ||||
| Current smokers | 197 (29.9%) | 69 (46.0%) | 128 (25.1%) | <0.001 |
| Former smokers | 156 (23.6%) | 11 (7.3%) | 145 (28.4%) | |
| Family history of stroke | 120 (18.2%) | 22 (14.8%) | 98 (19.2%) | 0.216 |
| National Institute of Health stroke scale † | 3 (2–6) | 4 (1–7) | 3 (2–6) | 0.027 |
| Minor stroke/transient ischemic attack | 250 (37.9%) | 63 (42%) | 187 (36.7%) | 0.237 |
| Major stroke | 410 (62.1%) | 87 (58.0%) | 323 (63.3%) | |
| Transthoracic echocardiography | ||||
| Left ventricular ejection fraction | 54.6 (7.3) | 58.6 (4.4) | 53.4 (7.5) | <0.001 |
| Evidence of wall motion abnormalities | 57 (8.6%) | 6 (4.0%) | 51 (10.0%) | 0.021 |
| Left ventricular hypertrophy | 242 (36.7%) | 20 (13.3%) | 222 (43.5%) | <0.001 |
| Left ventricular dilation | 42 (6.4%) | 2 (1.3%) | 40 (7.8%) | 0.004 |
| Transesophageal echocardiography | ||||
| Thrombus in left ventricle | 1 (0.2%) | 0 | 1 (0.2%) | 0.586 |
| Left atrial abnormalities | 105 (15.9%) | 2 (1.3%) | 103 (20.2%) | <0.001 |
| Left atrial/left atrial appendage thrombus | 19 (2.9%) | 1 (0.7%) | 18 (3.5%) | 0.065 |
| Thrombogenic milieu ‡ | 86 (13.0%) | 1 (0.7%) | 85 (16.7%) | <0.001 |
| Atrial septal abnormalities | 218 (33.0%) | 71 (47.3%) | 147 (28.8%) | <0.001 |
| Patent foramen ovale alone at rest | 56 (8.5%) | 21 (14%) | 35 (6.8%) | 0.006 |
| Patent foramen ovale alone after Valsalva | 90 (13.6%) | 30 (20%) | 60 (11.8%) | 0.01 |
| Patent foramen ovale and atrial septal aneurysm | 53 (8.0%) | 17 (11.3%) | 36 (7.1%) | 0.09 |
| Atrial septal aneurysm (no patent foramen ovale) | 19 (2.9%) | 3 (2%) | 16 (3.1%) | 0.464 |
| Aortic plaques ascendant and arch | 198 (30%) | 22 (14.7%) | 176 (34.5%) | <0.001 |
| Aortic plaques 1–3.9 mm | 85 (12.9%) | 15 (10%) | 70 (13.7%) | 0.231 |
| Aortic plaques ≥4 mm | 113 (17.1%) | 7 (4.7%) | 106 (20.8%) | <0.001 |
| Aortic arch thrombus | 11 (1.7%) | 1 (0.7%) | 10 (2.0%) | 0.278 |
⁎ Electrocardiographic findings on admission.
† Data are presented as median (interquartile range).
‡ Presence of spontaneous echocardiographic contrast in left atrium or left atrial appendage and/or low left atrial appendage velocity (<30 cm/s).
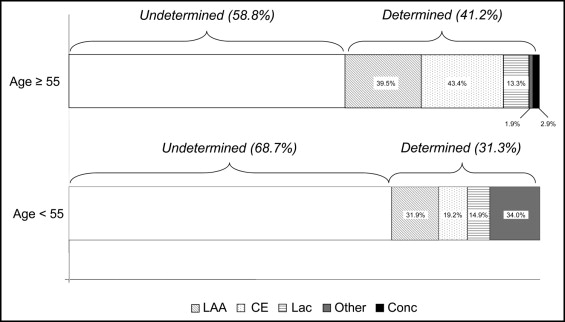
Overall, TEE detected 1 thrombus in the left ventricle, 105 left atrial abnormalities (46 in patients with sinus rhythm at admission), 218 (33.0%) atrial septal abnormalities, and 198 (30%) aortic arch and/or ascendant plaques ( Table 1 ). High-risk and potentially high-risk sources were detected using TEE in 308 patients (47%), of whom only 20 had more than one abnormality detected using TEE ( Table 2 ). The presence of left atrial/LAA thrombus, thrombogenic milieu, and aortic plaques prevailed in patients with a determined cause of stroke. In contrast, the prevalence of PFO alone at rest, and using the Valsalva maneuver, was significantly greater in patients with an undetermined cause of stroke ( Table 2 ). Among the patients with an undetermined cause of stroke, thrombogenic milieu and aortic plaques were more frequent in those aged ≥55 years. In contrast, among those with a determined etiology, the combination of PFO plus ASA was more prevalent in patients aged <55 years.
| Variable | Overall Population | Determined | Undetermined | |||
|---|---|---|---|---|---|---|
| Determined | Undetermined | Age <55 yrs | Age ≥55 yrs | Age <55 yrs | Age ≥55 yrs | |
| Patients (n) | 257 | 403 | 47 | 210 | 103 | 300 |
| High-risk sources | ||||||
| Thrombus in left ventricle | 0 | 1 (0.3%) | 0 | 0 | 0 (0) | 1 (0.3%) |
| Left atrial abnormalities | ||||||
| Left atrial/left atrial appendage thrombus | 15 (5.8%) ⁎ | 4 (1.0%) | 1 (2.1%) | 14 (6.7%) | 0 (0) | 4 (1.3%) |
| Thrombogenic milieu † | 60 (23.3%) ⁎ | 26 (6.5%) | 1 (2.1%) | 59 (28.1%) ⁎ | 0 (0) | 26 (8.7%) ‡ |
| Aortic plaques ≥4 mm and/or thrombus | 56 (21.8%) § | 57 (14.1%) | 5 (10.6%) | 51 (24.3%) § | 2 (1.9%) | 55 (18.3%) ⁎ |
| Potentially high-risk sources | ||||||
| Atrial septal abnormalities | ||||||
| Patent foramen ovale alone at rest | 14 (5.4%) | 42 (10.4%) § | 6 (12.8%) § | 8 (3.8%) | 15 (14.6%) | 27 (9%) |
| Patent foramen ovale and atrial septal aneurysm | 16 (6.2%) | 37 (9.2%) | 6 (12.8%) § | 10 (4.8%) | 11 (10.7%) | 26 (8.7%) |
| Potential risk sources | ||||||
| Atrial septal abnormalities | ||||||
| Patent foramen ovale alone after Valsalva | 22 (8.6%) | 68 (16.9%) ‡ | 6 (12.8%) | 10 (4.8%) | 27 (26.2%) ‡ | 41 (13.7%) |
| Atrial septal aneurysm (no patent foramen ovale) | 7 (2.7%) | 12 (3.0%) | 3 (6.4%) | 4 (1.9%) | 0 (0) | 12 (4.0%) § |
| Aortic plaques 1–3.9 mm | 37 (14.4%) | 48 (11.9%) | 5 (10.6%) | 32 (15.2%) | 10 (9.7%) | 38 (12.7%) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


