The association of a family history of peripheral arterial disease (PAD) with the presence of PAD is largely unknown. We conducted a case-control study of 2,296 patients with PAD (69 ± 10 years, 64% men) and 4,390 controls (66 ± 11 years, 62% men) identified from noninvasive vascular and stress testing laboratories at Mayo Clinic, Rochester, Minnesota, from October 2006 through June 2012. PAD was defined as an ankle brachial index of ≤0.9 at rest and/or after exercise, a history of lower extremity revascularization, or having poorly compressible leg arteries. Controls were patients with normal ankle brachial index or without a history of PAD. Family history of PAD was defined as having at least 1 first-degree relative who had undergone revascularization or stent placement for PAD before the age of 65 years. Logistic regression analyses were used to evaluate whether a family history of PAD was associated with the presence of PAD, independent of conventional risk factors. A family history of PAD was present more often in patients with PAD than in controls, with a resulting odds ratio (OR) of 2.20 (95% confidence interval [CI] 1.82 to 2.67). The association remained significant after adjustment for conventional risk factors (OR 1.97, 95% CI 1.60 to 2.42). The association was stronger in younger subjects (age <68 years; adjusted OR 2.46, 95% CI 1.79 to 3.38) than in older subjects (adjusted OR 1.61, 95% CI 1.22 to 2.12). A greater number of affected relatives with PAD was also associated with greater odds of presence of PAD (adjusted OR 1.86, 95% CI 1.48 to 2.33 and adjusted OR 2.56, 95% CI 1.60 to 4.11 for patients with 1 and ≥2 affected relatives with PAD, respectively). In conclusion, individuals with a family history of PAD have nearly double the odds of having PAD relative to those without such a history.
Highlights
- •
We conducted a large case-control study to investigate whether a family history of peripheral arterial disease (PAD) or coronary heart disease was associated with the presence of PAD.
- •
We showed that a family history of PAD as well as of coronary heart disease were each independently associated with the presence of PAD, the former being more strongly associated.
- •
In our study, parental or sibling history of PAD conferred similar odds.
- •
We found that the association was age dependent, being stronger in younger individuals, and the magnitude of the association was greater in those with a greater number of affected relatives.
Family history is a simple yet powerful clinical tool for improving risk assessment and thereby prevention of common chronic diseases. Although multiple studies have shown that a family history of coronary heart disease (CHD) is a significant risk factor for CHD, few studies have assessed whether a family history of peripheral arterial disease (PAD) is a risk factor for PAD. Small sample size and lack of power were major limitations of these studies, and family history as a risk factor for PAD has not been examined in a large cohort of patients with PAD. We therefore investigated whether a family history of PAD or CHD was associated with the presence of PAD in a large case-control study. We hypothesized that (1) a family history of PAD is a stronger risk factor for PAD than that of CHD and (2) a sibling history of PAD and/or CHD is a stronger risk factor than parental history because of greater sharing of environmental factors between siblings.
Methods
In October 2006, a biorepository of plasma and DNA of patients with PAD and controls was initiated by recruiting patients referred for lower extremity arterial evaluation to the Mayo Clinic noninvasive vascular laboratory and patients referred to the stress electrocardiographic laboratory to screen for CHD. From October 2006 to June 2012, a total of 10,206 patients were recruited. All participants gave their written consent for participation in the studies and the use of their data for future research. The study protocol was approved by the Institutional Review Board of the Mayo Clinic.
We defined PAD as having an ankle brachial index (ABI) of ≤0.9 at rest or 1 minute after exercise, the presence of poorly compressible arteries (defined as an average ABI in 1 leg >1.4 or lower extremity blood pressure of ≥255 mm Hg), or presence of ≥2 codes from a set of appropriate International Classification of Diseases, Ninth revision, Clinical Modification (ICD-9-CM) codes and a set of appropriate Current Procedural Terminology , fourth revision (CPT-4) codes. Possible PAD was defined as having only 1 code from the same set of appropriate ICD-9 and/or CPT-4 codes. The medical records of patients with possible PAD were manually reviewed by a trained physician (INI) to confirm their diagnosis. Patients who did not have PAD (presence of normal ABI or absence of the same set of appropriate ICD-9 and/or CPT-4 PAD codes) were classified as controls ( Figure 1 ). We excluded 144 patients who withdrew from the study after initial recruitment and those who identified themselves as adopted (n = 112). Several nonatherosclerotic vascular diseases can result in a low ABI, thereby mimicking atherosclerotic PAD; these include several vasculitides, Buerger’s disease, embolism, trauma to leg arteries, and other rare arteriopathies. Patients who had an abnormal ABI secondary to these conditions (n = 85) were excluded as well.
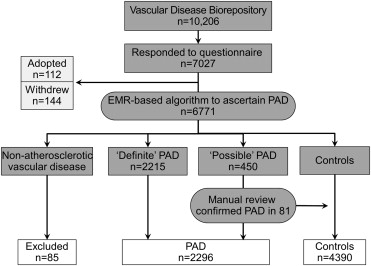
Family history of PAD was obtained using a questionnaire given to the patients at the time of recruitment; 7,027 patients (69%) returned the questionnaire. From this cohort, we identified 2,296 PAD cases and 4,390 controls ( Figure 1 ). Participants were asked about the presence of PAD and CHD in first-degree relatives, including mother, father, full siblings, sons, and daughters. A family history of PAD was considered to be present if a first-degree relative underwent lower extremity revascularization or stent placement before the age of 65 years. A family history of CHD was considered to be present if a first-degree relative had myocardial infarction, coronary revascularization, or stent placement before the age of 65 years.
Specific data elements of potential relevance to PAD were abstracted from the electronic medical record (EMR). These include birth date, gender, and race. Height, weight, and body mass index (BMI) closest to the index date (defined as the date of vascular laboratory evaluation or stress electrocardiographic testing at the time of recruitment) were also abstracted from the EMR directly.
Smoking status was ascertained from the study questionnaire, and “ever” smoking defined as having smoked >100 cigarettes. To ascertain other risk factors and co-morbidities, we used previously validated electronic algorithms that included ICD-9-CM codes, medication use, and laboratory data in the EMR. The presence of relevant ICD-9-CM codes up to 6 months after the index date was considered. Total cholesterol, high-, and low-density lipoprotein cholesterols, triglycerides, fasting blood sugar, and glycosylated hemoglobin were obtained from the laboratory database using a window of 1 year around the time of patient enrollment. Systolic and diastolic blood pressures at rest were obtained as structured observations from the EMR. The diagnosis of hypertension was established based on 2 blood pressure readings of ≥140/90 mm Hg within 3 months closest to the index date or a previous diagnosis of hypertension and current treatment with antihypertensive medication. Similarly, diabetes was diagnosed if a patient had a fasting plasma glucose level of ≥126 mg/dl, a random glucose level of >200 mg/dl, hemoglobin A1c of >6.5%, or had a previous diagnosis and was on treatment with oral hypoglycemic agent(s) or insulin. Dyslipidemia was defined as a total cholesterol level of >220 mg/dl, a high-density lipoprotein cholesterol level of <40 mg/dl (in men) and <45 mg/dl (in women), triglycerides >200 mg/dl, or the use of a lipid-lowering medication.
Continuous data are summarized as either mean ± SD or median and quartiles. Between-group differences were assessed by an unpaired 2-tailed Student t test or the Wilcoxon rank sum test. Categorical data are expressed as percentages, and between-group differences were assessed by the chi-square test statistic. We constructed multivariate logistic regression models that adjusted for conventional risk factors and other potential confounding variables to assess whether a family history of PAD or CHD was independently associated with PAD. Adjustments were performed for age and gender, BMI, smoking history, diabetes, hypertension, and dyslipidemia. To compare the association of a parental and sibling history of PAD and CHD with the presence of PAD, we stratified study participants based on family history (parental or sibling history) and repeated analyses described previously for each group separately. We also checked for interactions between conventional risk factors and family history in the prediction of PAD. A 2-sided p value <0.05 was deemed statistically significant. Statistical analyses were carried out using the SAS, version 9.1 (SAS Institute, Cary, North Carolina), software package.
Results
Participant characteristics are listed in Table 1 . Patients with PAD were older, and the proportion of men was similar in both groups. Patients with PAD had a greater prevalence of increased BMI, dyslipidemia, diabetes, history of smoking, and hypertension ( Table 1 ).
| Variables | PAD (n = 2296) | Control (n = 4390) | p Value |
|---|---|---|---|
| Age (years) | 69.0 ± 10.4 | 66.2 ± 11.1 | <0.001 |
| Men | 1457 (63.5%) | 2713 (61.8%) | 0.18 |
| BMI (kg/m 2 ) | 29.3 ± 5.5 | 28.7 ± 5.3 | <0.001 |
| Dyslipidemia | 1815 (79.1%) | 3043 (69.3%) | <0.001 |
| Diabetes mellitus | 834 (36.3%) | 701 (16.0%) | <0.001 |
| Ever smoked | 1841 (80.2%) | 2197 (50.0%) | <0.001 |
| Hypertension | 1517 (66.1%) | 2077 (47.3%) | <0.001 |
| Number of first degree relatives with PAD | |||
| 0 | 2058 (89.6%) | 4171 (95.0%) | <0.001 |
| 1 | 190 (8.3%) | 184 (4.2%) | |
| 2 | 30 (1.3%) | 22 (0.5%) | |
| ≥3 | 18 (0.8%) | 13 (0.3%) | |
| Number of first degree relatives with CHD | |||
| 0 | 933 (40.6%) | 1934 (44.1%) | <0.001 |
| 1 | 706 (30.7%) | 1497 (34.1%) | |
| 2 | 405 (17.6%) | 633 (14.4%) | |
| ≥3 | 252 (11.0%) | 326 (7.4%) | |
Prevalence of a family history of PAD was significantly greater in patients with PAD than in controls (10.4% vs 5.0%, respectively, p <0.0001; Table 2 ). Both sibling and parental histories of PAD were more often present in patients with PAD than controls (7.3% vs 3.4%, p <0.0001 and 2.9% vs 1.3%, p <0.0001, respectively). In univariate logistic regression analysis, a family history of PAD was associated with a higher odds ratio (OR) of 2.20 (95% confidence interval [CI] 1.82 to 2.67) of having PAD. Multivariate logistic regression analysis showed that the association of a family history of PAD with having PAD was only modestly attenuated and remained significant after adjustment for age, gender, BMI, ever smoking, diabetes, hypertension, and dyslipidemia (OR 1.97, 95% CI 1.60 to 2.42; Figure 2 ). Similar results were observed for the sibling and parental history of PAD (OR 1.86, 95% CI 1.46 to 2.38 and OR 2.30, 95% CI 1.56 to 3.39, respectively). We noted a significant (p <0.01) interaction between age and family history of PAD in predicting the presence of PAD. The interaction indicated that the association between family history and presence of PAD was stronger in younger subjects. Therefore, we also performed subgroup analyses stratifying by the median age (68 years) of the entire cohort. The associations were stronger in the younger age group ( Figure 2 ). The adjusted ORs of having PAD were 2.46 (95% CI 1.79 to 3.38) and 1.61 (95% CI 1.22 to 2.12), in the younger and older age group, respectively.
| PAD (n = 2296) | Control (n = 4390) | OR (95% CI) | |
|---|---|---|---|
| Family history of PAD | 238 (10.4%) | 219 (5.0%) | 2.20 (1.82–2.67) |
| Sibling history of PAD | 168 (7.3%) | 151 (3.4%) | 2.22 (1.77–2.78) |
| Parental history of PAD | 66 (2.9%) | 61 (1.3%) | 2.10 (1.48–2.99) |
| Younger (Individuals <68 y) | PAD (n = 941) | Control (n = 2362) | OR (95% CI) |
|---|---|---|---|
| Family history of PAD | 112 (11.9%) | 101 (4.3%) | 3.02 (2.28–4.00) |
| Sibling history of PAD | 68 (7.2%) | 56 (2.4%) | 3.21 (2.23–4.61) |
| Parental history of PAD | 51 (5.4%) | 44 (1.9%) | 3.02 (2.00–4.55) |
| Older (Individuals ≥68 y) | PAD (n = 1355) | Control (n = 2028) | OR (95% CI) |
|---|---|---|---|
| Family history of PAD | 126 (9.3%) | 118 (5.8%) | 1.66 (1.28–2.15) |
| Sibling history of PAD | 100 (7.4%) | 95 (4.7%) | 1.62 (1.21–2.17) |
| Parental history of PAD | 15 (1.1%) | 17 (0.8%) | 1.32 (0.66–2.66) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


