Accelerated cardiovascular pathology in the setting of renal failure
Despite continued advancements in understanding and managing CVD and ESRD, we simply do not fully understand the intersection of these often co-morbid diseases [9]. We have recognized for some time LVH is prevalent in ESRD patients on hemodialysis. Predictors of HF in the ESRD population include primarily co-morbidities of CVD: history of ischemic heart disease, LVH, diabetes mellitus, age >60 years, heightened inflammation (defined as elevated C-reactive protein), and >1 year on dialysis [10, 11]. However, some may be promoted in the setting of dialysis.
Although dialysis reduces fluid overload, it also induces significant hemodynamic stress, inflammation, and endothelial dysfunction. Cardiovascular event incidence is high in the first few weeks after hemodialysis initiation [12] and repeated treatment promotes physiologic changes that may inadvertently contribute to the accelerated decay of cardiac function. Indeed, heart failure with preserved ejection fraction (HFpEF) is the more prevalent subtype in hemodialysis patients, [13, 14] and age, female sex, body mass index, blood pressure, and dialysis modality are predictive of HFpEF [13].
Cardiovascular Stressors of Hemodialysis
Pathophysiologic Considerations
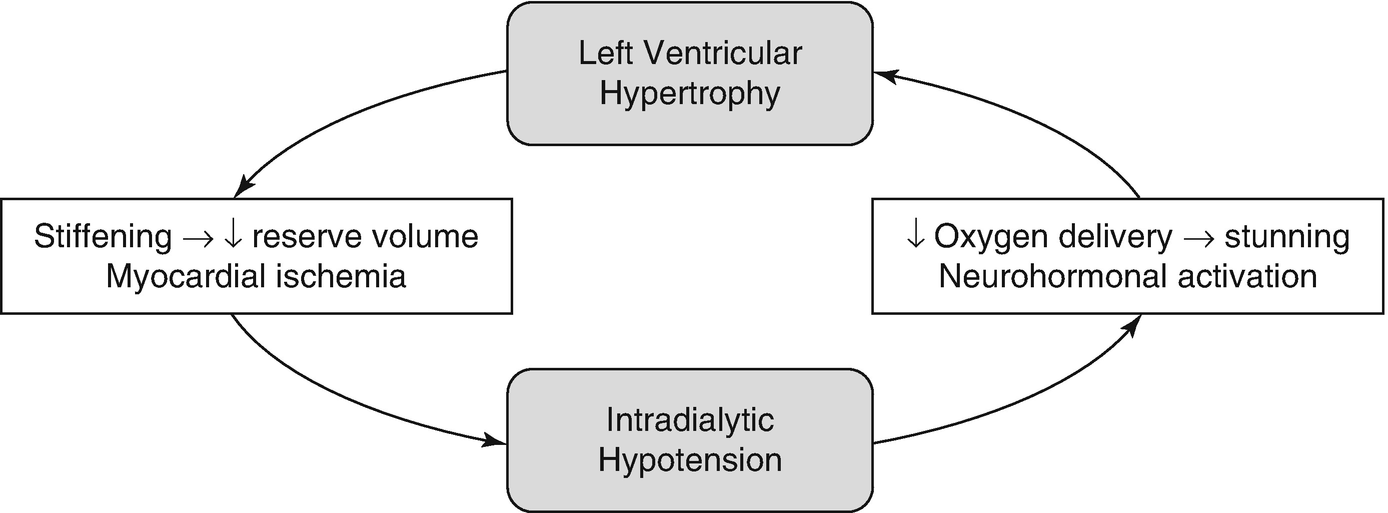
Vicious cycle for myocardial damage during dialysis
During dialysis, oxygen delivery to the subendocardium is compromised due to both decreased supply (decreased blood volume, pre-existing anemia) and increased demand (neurohormonal activation, continuous ultrafiltration). In addition, left ventricular hypertrophy and uremic cardiomyopathy contribute to compression of the coronary vasculature during systole [18]. Hemodialysis-induced regional wall motion abnormalities occur in over 25% of patients and are an independent risk factor for increased mortality [19]. Patients often develop myocardial stunning during dialysis and this injury often remains subclinical, particularly in patients with diabetic neuropathy [19, 20]. However, direct demonstration that such observations indeed correspond to the oxygen tension at the myocyte level have not been consistently shown in humans, even though such postulated pathophysiology is highly plausible.
Extrinsic stressors not accounted for in non-dialysis individuals may also play a role. For example, dialysis also imposes thermal stress with relatively large volume infusion and exchange of dialysates, and an increase in core temperature can be observed during a typical session [21]. Heat accumulation is related to decline in blood volume, which can lead to paradoxical reflex vasodilation. Especially in those with large ultrafiltration requirements, this vasodilation may prevent maintenance of blood pressure. Cooling of the dialysate has been shown to decrease the incidence of IDH compared to isothermic dialysis, in which there is no change in core temperature, and thermoneutral dialysis in which there is no energy added or removed from the patient [22].
Dialysis also imposes inflammatory stress, most notably due to biomaterial contact. Current dialyzer membranes, optimized for pore size manipulation and permeability, are hydrophobic and provide ample surface area for protein deposition, which promotes deposition of IgG, C3, and fibrinogen, promoting complement activation and coagulation [23]. Complement activation has been shown to occur in the first 30 minutes of HD sessions and leads to proinflammatory changes in cytokine transcription profiles [23, 24]. Heparin can inhibit complement, however, this effect is not seen in the doses typically prescribed during dialysis [23]. Inflammatory markers, including C-reactive protein (CRP), have been independently associated with HD-induced regional left ventricular systolic dysfunction [19]. The mechanistic link between inflammation and CVD is not clear and involves significant crosstalk between inflammation, thrombosis, and vascular dysfunction pathways [25]. Surface-modified hydrophilic membranes which have lower protein-adsorptive properties are available but not commonly used [23, 26].
Evaluation of Congestive HF in ESRD
Renal Evaluation in ESRD with HF
Dialysis modality and timing can impact the severity of dialysis-induced cardiovascular dysfunction. Interestingly, long-term development of LVH is more common and more severe in peritoneal dialysis (PD) patients, while hemodialysis (HD) patients may have more acute issues with effective blood pressure control. Standard intermittent hemodialysis is typically three sessions per week lasting 4–6 hours. In patients with persistent symptoms, often times a re-evaluation of their “dry weight” targets is necessary with changes in cardiovascular physiology. This can be accomplished by assessment of hemodynamics to determine if intracardiac filling pressures are adequately controlled (as a surrogate of volume overload). Prolonging duration of dialysis with slower fluid removal rates and more frequent sessions may reduce cardiovascular stress and improve survival. A benefit in a composite outcome of death, LV mass, and quality of life was associated with intensive hemodialysis in the Frequent Hemodialysis Network (FHN) Daily trial [27]. Meanwhile, online hemodiafiltration also showed improved survival over high-flux hemodialysis in the ESHOL (Estudio de Supervivencia de Hemodiafiltración On-Line) study [28]. Alternatively, home dialysis may also achieve the same duration and fluid removal goals, and has gained some traction for patients capable of performing it at home.
Dialysis access also directly or indirectly contributes to cardiovascular dysfunction. An arteriovenous fistula (AVF) is the most common route of vascular access for chronic hemodialysis patients due to high blood flow rate, patency, and low infection risk. Upon creation of the direct shunt from arterial to venous circulation, systemic vascular resistance is decreased. In compensation, renin angiotensin aldosterone and sympathetic systems are activated, ultimately increasing cardiac output. Neurohormonal activation promotes cardiac remodeling and further left ventricular hypertrophy. Congruently, patients with AVF closure show a decrease in both eccentric and concentric hypertrophy [29]. Patients with underlying heart disease may not be able to sustain compensatory mechanisms and cardiac remodeling requirements, making them more susceptible to developing HF with a patent AVF. In the setting of high AVF blood flow, some patients can develop symptomatic high-output HF, defined by symptoms of HF in the presence of an elevated cardiac index (≥3 L/min2) and low systemic vascular resistance [30]. This can be demonstrated during right heart catheterization, in which temporary compression of AVF by a blood pressure cuff can acutely reverse these hemodynamic abnormalities and indicate the need to surgically revise the AVF. In a small randomized study, ligation of the hemodialysis AVF in stable post–renal transplant patients improves LV remodeling and also reduces NT-proBNP at 6 months [31].
Cardiovascular Evaluation in ESRD with HF
Clinicians should actively monitor signs and symptoms of HF in all ESRD patients, which is the most challenging aspect of making the diagnosis since many of the clinical presentation of HF and ESRD are similar (shortness of breath, fatigue, edema, exercise intolerance). All ESRD patients at risk of congestive HF should have baseline echocardiographic assessment at dry weight. This is a challenge upon itself since body composition changes over time and so does the evolution of “dry weight” that is hard to determine at the bedside. Although no specific guideline recommendations are available, it is reasonable for symptomatic patients with LVEF >35% to have serial transthoracic echocardiography for monitoring annually, while those with LVEF ≤35% should repeat every 3–6 months until stabilized if correctable abnormalities are present especially when there are treatment changes with medical optimization [32]. Echocardiography is mainly a monitoring and diagnostic tool, and not a treatment modality.
Over time with volume overload in a similar manner to left-sided valve diseases or HFpEF, left-sided HF progresses to right-sided HF as increased pressures are transmitted to the pulmonary circuit. Persistent PH promotes pathological changes in the vasculature, including intimal thickening, smooth muscle cell hypertrophy, and development of irreversible plexiform lesions. Fluid overload between dialysis sessions can also cause progressive PH. Beyond the above-mentioned indications of right heart catheterization for assessing volume status and determining excessive shunting from AVF, hemodynamic evaluation is often helpful to determine the severity of PH and determinination of adequate cardiac compensation and balance between left- and right-sided pressures and the need for pharmacologic interventions or changes in dialysis modalities or goals.
Interestingly, the goals of dialysis have remained largely unchanged over the decades, which are focused on reducing solutes rather than other uremic toxins or maintaining optimal vascular function. Future studies are warranted to determine the optimal targets and choices of dialysis modalities and dialysate management.
Medical Management of Congestive HF in ESRD
Heart Failure Disease Management
Managing congestive HF in the ESRD population follows the same general principles as in the rest of the population, however, there is a paucity of data supporting clinical decisions. In HFpEF, evidence is even more limited but generally the goal is management of contributing conditions. The primary targets are adequate volume and afterload reduction. Dialysis remains a mainstay for acute decompensation from a volume management perspective (many patients on dialysis are oliguric or even anuric). In the long-term, salt restriction, beta blockers, and angiotensin-converting enzyme inhibitors (ACE-I) or angiotensin receptor blockers (ARB) can be used as treatment when there is adequate blood pressure support. In the context of HFrEF, addition of ARB like telmisartan to standard therapies significantly reduces all-cause mortality, cardiovascular death, and HF hospital stays in hemodialysis patients, however, this has not been shown in HFpEF [33]. ARB therapy can reduce the risk of HF in ESRD patients but ACE-I is generally preferred [34]. Interestingly, there have been suggestions that pre-dialysis patients with Stage 4 CKD may also benefit from ACE-I [35].
On the other hand, beta-blockers mitigate deleterious effects of neurohormonal activation, with carvedilol, which is poorly dialyzed, specifically showing significant benefit in ESRD patients [36]. Large-scale clinical trials using beta-blockers have been attempted, but recruitment and blood pressure tolerability have been challenging [37, 38]. Meanwhile in small studies, spironolactone has been shown to reduce blood pressure, aortic calcification, and mortality [39, 40]. The use of mineralocorticoid receptor antagonists (MRA) remains controversial and randomized controlled-trials are ongoing (ALdosterone Antagonist Chronic HEModialysis Interventional Survival Trial [ALCHEMIST], ClinicalTrials.gov NCT01848639). It is noted that the combination of ACE-I, ARB, and an MRA should be avoided due to concern for hyperkalemia.
Associated conditions include atrial fibrillation, hyperlipidemia, and myocardial ischemia. The patient should be assessed for comorbid atrial fibrillation, which is common in HFpEF, with an EKG. Restoration and maintenance of sinus rhythm is preferred but rate control can be targeted when this cannot be achieved. Carvedilol, which shows significant benefit in ESRD patients, would be a reasonable first-line option although there might be higher risk of hypotension [37, 38]. Though used in HFrEF patients, digoxin use in HFpEF patients with AF at a mean follow-up of 37 months had no effect on all-cause or cause-specific mortality or all-cause or cardiovascular hospitalization [41]. Additionally, digoxin use in dialysis patients has been associated with increased mortality, particularly if predialysis serum potassium levels are low [42].
Coronary Atherosclerotic Disease Management
Statins appeared to have no incremental benefit in preventing major adverse cardiac events in the ESRD population as addressed in the 4-D, AURORA, and SHARP trials, though continuation of previously prescribed statin therapy according to latest clinical guidelines is appropriate [43–46]. In ESRD patients with coronary artery disease, antithrombotic agents are often prescribed with little evidence to guide decision-making, and with increased complications since ESRD patients have acquired intrinsic platelet abnormalities. Specifically, ESRD patients have reduced serotonin content and impaired ADP release, and are simultaneously at increased risk of bleeding and in a prothrombotic state with “nontraditional” risk factors for thrombosis, such as hyperhomocysteinemia, endothelial dysfunction, inflammation, and malnutrition [47, 48].
Assessment of coronary artery disease is difficult in the ESRD population due to complications from comorbid conditions, such as diabetic neuropathy. However, revascularization by percutaneous coronary intervention (PCI) should be pursued if indicated. Low-dose aspirin use in ESRD patients is of unproven cardiovascular benefit and no clinical trials have addressed its utility. Observational studies inherently present confounding by indication but have reported increased mortality risk in hemodialysis patients on aspirin. Overall, usage is likely safe, provider-dependent, and may be discussed with the patient [49–51]. Meanwhile, the Arterial Revascularization Therapies Study (ARTS) showed that in patients with CKD, there was no significant difference in operative death, myocardial infarction, and stroke for those treated by either coronary artery bypass grafting (CABG) or PCI, while CABG was associated with a lower risk for repeat revascularization [52]. For patients with Stage IV chronic kidney disease, CABG was associated with a decreased mortality rate compared to PCI, even though there may be higher mortality rates for the first 3 months in patients who underwent CABG compared to PCI [53]. The ASCERT study showed that the estimated mortality rate in the general population who underwent CABG was 3.2%, 6.4%, 8.1%, and 23.3% at 30 days, 180 days, 1 year, and 3 years, respectively [53]. Predictors for late cardiac events include advanced age (>63 years), diabetes, and peripheral artery disease, whereas, predictors for late death include diabetes and LVEF <40% [54].
Anemia and Metabolic Management
To mitigate chronic myocardial ischemia, anemia can be managed using Erythropoietin-Stimulating Agents (ESA), which effectively increase hemoglobin levels. However, higher doses of ESA and higher hematocrit management goals have failed to show survival benefit in multiple RCTs; secondary analyses of these trials has implicated high ESA dose or resistance, rather than higher hemoglobin levels, as the cause [55–57]. Erythropoietin-stimulating agents are also associated with a higher risk of thrombotic events, mediated through a multifaceted mechanism of polycythemia/hyperviscosity syndrome, thrombocytosis, platelet hyperactivity, and activation of blood coagulation [58].
Both hyperphosphatemia and hyperparathyroidism promote vascular calcification in ESRD patients; the goal of management is to treat hyperphosphatemia, maintain normocalcemia, and treat vitamin D deficiency. Phosphate binders, calcimimetics, and vitamin D analogs can be used. Both ergocalciferol and cholecalciferol are effective in treating vitamin D deficiency. Metabolic acidosis can be managed using sodium bicarbonate. Dialysate content can also be manipulated to control electrolyte balance and affect survival. A dialysate potassium of <2 mEq/L has been associated with an increased risk of sudden cardiac death and a lower serum-to-dialysate calcium gradient may be advisable in patients with cardiorenal syndrome [59].
Device Therapy for HF and ESRD
ESRD patients are generally managed medically due to higher rates of complications, however, implantable cardioverter defibrillator (ICD) therapy may be considered in patients with cardiomyopathy as primary prevention of sudden cardiac death. The benefits and risks of ICD should be discussed with patients with LVEF consistently <35% despite medical therapy. Compared with estimates in HFrEF, the rates of sudden cardiac death in HFpEF are lower [60]. However, ESRD patients, who inherently have higher arrhythmic risk, may benefit from ICD therapy. In patients with mild to moderate CKD, ICD implantation regardless of indication reduces mortality, however, this benefit is balanced with a higher procedural risk in patients with more advanced renal failure [61]. Meanwhile, for those with CKD eligible for cardiac resynchronization therapy, they should be considered as they provide incremental benefits compared to ICD alone [62].
Renal Transplantation Candidacy and Considerations
Patients with HF are often not referred for renal transplantation for a wide range of reasons. However, renal transplantation has been shown to improve left ventricular mass and function [63, 64]. Prolonged dialysis is associated with a decrease in the beneficial effect of transplantation, thus renal transplantation should be considered early in patients at risk of, or already diagnosed with congestive HF. There is currently no consensus on the minimum LVEF for renal transplantation, and it is important to reassess eligibility after optimizing medical therapy.
Combined heart-kidney transplants have outcomes similar to those with primary heart transplantation, though these cases are highly selective and uncommon. About a decade ago, a risk score was constructed from the United Network for Organ Sharing (UNOS) data that identified risk factors associated with worse survival benefit included: (1) a history of peripheral arterial disease (4 points); (2) recipient age >65 years (3.5 points); (3) non-ischemic cause of heart failure (2 points); (4) bridge to transplantation with use of a ventricular assist device (2 points); and dialysis dependency (2.5 points) [65]. They observed in patients with eGFR less than 33 mL/min undergoing heart trasnplant with low-risk (total score <4), there was a significant survival benefit of combined heart-kidney transplant compared to heart transplant alone. Newer analysis also extended such benefits to those at high risk of developing post-transplant dialysis dependence or in older individuals [66, 67].
Treatment Pearls for the Case Vignette
This patient’s echocardiogram supports a diagnosis of HFpEF with diastolic dysfunction and PH. The history of worsening symptoms suggests chronic progression, which is common in ESRD patients. High-output failure due to AVF is of lower probability in this case due to the chronicity of dialysis, although it needs to be ruled out if accompanied by acute changes in the AVF itself. In this patient with PH, prevention of progression to right-sided HF should be prioritized. A right heart catheterization would be necessary to determine the degree of elevated pulmonary pressures and their reversibility, as well as the severity of uremic cardiomyopathy. The challenge of medication intolerance due to reducing perfusion pressures is a common challenge, and there are various means including holding drug doses before/after dialysis session or changing to shorter acting drugs with lower propensity of hypotension. In this case with HFpEF, the need to maintain neurohormonal antagonists is less certain. If feasible, switching to frequent hemodialysis may likely provide benefit in improving left ventricular mass and survival. As discussed, the FHN found benefit in a composite outcome of death, LV mass, and quality of life with intensive hemodialysis [27]. Hemodiafiltration has also shown a trend towards improved survival over standard hemodialysis [28]. Home dialysis is another option, but the cost and logistic inconvenience of daily home dialysis may be a barrier for some patients. The patient’s advanced age and the diagnosis of HFpEF precluded any meaningful indications or considerations of invasive device therapies or transplantation options, although evaluation of underlying ischemic causes that leads to such changes in clinical presentation may be warranted.

Full access? Get Clinical Tree


