Myeloperoxidase (MPO) is associated with risk in acute coronary syndromes. However, the precise role it plays in ST-elevation myocardial infarction (STEMI) remains unclear. In this study we tested the hypothesis that levels of MPO in plasma after a myocardial infarction are affected by its ability to bind to the endothelium and there is local release of the enzyme at the culprit lesion. We measured plasma MPO in systemic circulation and throughout the coronary circulation in patients with STEMI undergoing primary percutaneous coronary intervention (PCI). MPO levels at the femoral artery were higher (p <0.001) in patients with STEMI (n = 67, median 45 ng/ml, interquartile range 34 to 83) compared to control patients (n = 12, 25 ng/ml, 19 to 30) with chronic stable angina undergoing elective PCI. After administration of the anticoagulant bivalirudin in 13 patients with STEMI, plasma MPO was increased only at the culprit coronary artery lesion before PCI (178 ng/ml, 91 to 245) versus all other sites (femoral artery 86 ng/ml, 54 to 139, p = 0.019). Administration of heparin caused a marked increase of plasma MPO. Even so, it was still possible to detect an increase of plasma MPO at culprit lesion in patients with STEMI (n = 54, 171 ng/ml, 122 to 230) versus controls (n = 12, 136 ng/ml, 109 to 151, p <0.05) after heparin and before PCI. MPO levels were higher at the culprit lesion in patients with STEMI who presented early and in those with restricted flow (p <0.05). In conclusion, our results demonstrate that, in addition to a systemic increase of MPO in patients presenting early with STEMI, levels of this leukocyte enzyme are increased at the culprit coronary lesion before PCI.
We hypothesized that in patients with acute ST-segment elevation myocardial infarction (STEMI) who present early, in addition to a systemic effect, plasma myeloperoxidase (MPO) levels may be increased locally at the culprit lesion within the occluded coronary artery. Blood was sampled peripherally before administration of anticoagulant and at sites throughout the coronary circulation during primary percutaneous coronary intervention (PCI). Initially we investigated levels of plasma MPO in a subgroup of patients with STEMI using the direct thrombin inhibitor bivalirudin, which does not affect release of MPO from the endothelium. We then assessed levels of plasma MPO in a larger group of patients with STEMI who received heparin. To control for the effects of heparin, we compared the results in the STEMI group to a group of patients with chronic stable angina (CSA) undergoing elective PCI, after similar doses of weight-adjusted heparin.
Methods
The study was approved by the New Zealand Ministry of Health Upper South A regional ethics committee (ethics reference URA/05/08/097). All patients gave written informed consent before enrollment. Patients with acute STEMI presenting to Christchurch Hospital were considered eligible if symptoms suggesting acute myocardial ischemia lasted >30 minutes, onset of symptoms was <12 hours previously, ST-segment elevation was >0.1 mV in ≥2 leads on electrocardiogram, and had no contraindications to primary PCI. Exclusion criteria were left main coronary artery disease, stent thrombosis, rescue PCI after thrombolysis, or cardiogenic shock. Patients were pretreated with aspirin 300 mg and clopidogrel 600 mg.
Blood was drawn peripherally from the femoral artery sheath before administration of anticoagulant. Further sampling was carried out before angioplasty and stenting in the ascending aorta at the coronary ostium through a guiding catheter and from the coronary sinus using a separate catheter introduced through the femoral vein. The culprit lesion within the coronary artery was sampled after careful passage of the guidewire across the lesion and use of a low-profile sampling catheter (multifunctional probing catheter; Boston Scientific Corp., Natick, Massachusetts) at the site of occlusion, before further mechanical intervention. Before sampling, baseline flow in the culprit coronary artery was recorded using Thrombolysis In Myocardial Infarction (TIMI) grade flow, with patients grouped into TIMI 0 to 1 (absent or little) flow or TIMI 2 to 3 (almost normal or normal) flow. Figure 1 depicts the multifunctional probing catheter sampling blood from the culprit coronary lesion within the coronary artery. After the culprit lesion was sampled, standard primary PCI using aspiration thrombectomy and coronary stenting was performed. After successful PCI, further blood samples were drawn at the culprit lesion with the same sampling catheter, coronary sinus, and aorta at the coronary ostium. A follow-up sample was drawn from a forearm vein 24 hours after PCI. All samples were collected into tubes containing ethylenediaminetetra-acetic acid and placed on ice immediately. Whole blood was centrifuged at 1,200 g at 4°C for 10 minutes. Plasma was separated and stored at −80°C for subsequent analysis.

Twelve patients with Canadian Cardiovascular Society class II to III angina symptoms and documented stable coronary disease undergoing elective single-vessel PCI were studied as a control. All patients had a plasma troponin I level <0.01 before PCI. The blood sampling protocol was identical to that for the STEMI group. Patients were excluded if they had active infection, acute inflammatory disease, malignancy, or chronic kidney disease.
Plasma MPO was measured by sandwich enzyme-linked immunosorbent assay and results expressed in nanograms per milliliter. Based on a molecular weight of 145,000 g/mol for MPO, these values can be converted to picomoles per liter by multiplying them by a factor of 7. Control and patient samples were included on each plate. Plasma samples were diluted 1:10 and MPO was captured using a monoclonal antibody (Abcam, Cambridge, United Kingdom). It was detected using a rabbit polyclonal antibody produced in house, and goat anti-rabbit immunoglobulin G containing a biotin conjugate, which bound avidin coupled to alkaline phosphatase. When 5 different plasma samples were spiked with purified MPO 60 ng/ml, 99 ± 6% of the total was detected. Detection of MPO in plasma by enzyme-linked immunosorbent assay is not affected by administration of heparin to patients because previously we found that in a separate group of patients that there was no association between MPO levels in plasma and heparin treatment. Plasma leukocyte elastase was measured using a commercial kit (Immunodiagnostik, Bensheim, Germany). Plasma samples were diluted 1:100 with assay buffer then bound, and unbound elastase was measured by enzyme-linked immunosorbent assay.
Whole blood was obtained from healthy controls (n = 5) and treated with heparin or bivalirudin in vitro to determine whether either anticoagulant caused release of MPO from neutrophils. Concentrations of anticoagulant varied over their pharmacologic ranges (heparin 0 to 15 IU/ml, bivalirudin 0 to 0.1 mg/ml). After 30 minutes of incubation at 37°C, whole blood was centrifuged, plasma extracted, and MPO assayed using the enzyme-linked immunosorbent assay described earlier. Neutrophils were isolated by Ficoll-Hypaque centrifugation, dextran sedimentation, and hypotonic lysis of red blood cells as previously described. These were also treated with the anticoagulants as described earlier. After 30-minute incubation, cells were pelleted by centrifugation and supernatants analyzed for MPO.
Data were analyzed using nonparametric statistics and reported as median and interquartile range unless otherwise stated. Mann-Whitney rank sum test was used for comparing 2 groups. Friedman repeated measures analysis of variance on ranks was used to determine differences for multiple comparisons. Student-Newman-Keuls method was then used to identify groups that differed from each other. Pearson chi-square test was used for categorical variables. Relation between demographic variables and plasma MPO was assessed using forward stepwise linear regression. Differences with a p value <0.05 were accepted as statistically significant.
Results
Sixty-seven patients with acute STEMI treated by primary PCI received anticoagulant after sampling from the femoral artery sheath. Baseline demographics of the 3 groups are listed in Table 1 .
| Variable | STEMI and Bivalirudin | p Value ⁎ | STEMI an Heparin | Elective PCI and Heparin | p Value † |
|---|---|---|---|---|---|
| (n = 13) | (n = 54) | (n = 12) | |||
| Men | 10 (76%) | NS | 35 (65%) | 8 (67%) | NS |
| Age (years) (age range) | 63 (42–85) | NS | 61 (36–88) | 68 (49–79) | 0.11 |
| Diabetes mellitus | 3 (23%) | NS | 15 (28%) | 4 (33%) | NS |
| Hypertension | 8 (62%) | 0.07 | 21 (39%) | 11 (92%) | 0.001 |
| Hypercholesterolemia ‡ | 9 (69%) | NS | 26 (48%) | 11 (92%) | 0.009 |
| Smoker | 9 (69%) | NS | 34 (63%) | 4 (33%) | 0.14 |
| Preceding angina | 5 (38%) | NS | 14 (26%) | 12 (100%) | <0.001 |
| Statin therapy | 1 (8%) | NS | 7 (13%) | 10 (83%) | <0.001 |
| Angiotensin-converting enzyme inhibitor therapy | 3 (23%) | 0.18 | 5 (9%) | 8 (67%) | <0.001 |
| β-blocker therapy | 3 (23%) | NS | 8 (15%) | 9 (75%) | <0.001 |
| Baseline leukocyte count 10 9 /L, mean ± SD | 11.35 ± 4.56 | NS | 10.53 ± 4.51 | 6.28 ± 0.90 | 0.01 |
| Culprit artery | |||||
| Left anterior descending coronary artery | 6 (46%) | NS | 23 (43%) | 10 (83%) | 0.006 |
| Time to presentation (minutes) | |||||
| 0–90 | 4 (31%) | 0.06 | 4 (7%) | ||
| 90–180 | 2 (15%) | NS | 26 (48%) | ||
| >180 | 7 (54%) | NS | 24 (44%) | ||
| Baseline Thrombolysis In Myocardial Infarction grade flow | |||||
| 0–1 | 13 (100%) | 0.03 | 39 (72%) | ||
| 2–3 | 0 | 15 (28%) | |||
| Final Thrombolysis In Myocardial Infarction grade 2–3 flow | 13 (100%) | NS | 52 (96%) | 12 (100%) | NS |
| Heparin (IU/kg), median (interquartile range) | NA | 85.3 (77–89) | 83.3 (83–86) | NS |
⁎ Patients with STEMI treated with bivalirudin compared to those treated with heparin.
† Patients with STEMI given heparin compared to those with elective PCI (control group) given heparin.
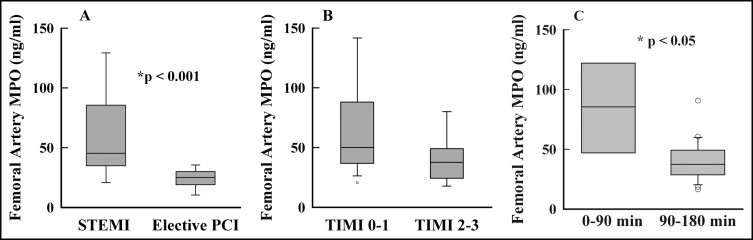
Femoral artery plasma MPO, before anticoagulants, was higher (p <0.001) in patients with STEMI (n = 67, median 45 ng/ml, interquartile range 34 to 83) compared to control with CSA (n = 12, 25 ng/ml, 19 to 30; Figure 2 ). To determine whether femoral artery plasma MPO levels were influenced by baseline flow in the culprit coronary artery, we categorized patients with STEMI into those with TIMI grade 0 to 1 flow and those with TIMI grade 2 to 3 flow before PCI. Patients with STEMI and TIMI grade 0 to 1 flow had higher (p <0.05) plasma MPO (n = 42, 50 ng/ml, 37 to 87) compared to those with TIMI grade 2 to 3 flow (n = 11, 37 ng/ml, 24 to 48) ( Figure 2 ). Furthermore, patients with STEMI who presented within 0 minute to 90 minutes from onset of symptoms of chest pain had higher levels of plasma MPO (p = 0.002) compared to those presenting within 90 to 180 minutes ( Figure 2 ).
Bivalirudin was given after sampling from the femoral artery. Before bivalirudin, this subgroup of patients with STEMI had increased plasma MPO (p <0.01) at the femoral artery (n = 13, 86 ng/ml, 54 to 140) compared to controls with CSA (n = 12, 25 ng/ml, 19 to 29). This result was consistent with their baseline TIMI grade 0 to 1 culprit coronary artery flow. Levels of MPO in plasma sampled from the aorta and coronary sinus before PCI were similar to those in plasma from the femoral artery ( Figure 3 ). However, plasma MPO levels at the site of the culprit coronary lesion (n = 13, 178 ng/ml, 91 to 245) were significantly increased (p = 0.019) compared to those in plasma from the femoral artery (n = 13, 86 ng/ml, 54 to 139) and all other sites (p <0.05; Figure 3 ). This result demonstrates that levels of plasma MPO at the culprit lesion were higher before PCI than after the intervention.
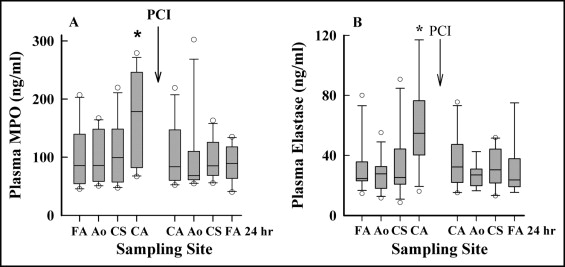
Leukocyte elastase (LE), like MPO, is derived from the primary granules of neutrophils. Its plasma levels mirrored those of MPO in patients receiving bivalirudin and were higher at the culprit coronary lesion before PCI (p = 0.014) compared to all other sampling sites ( Figure 3 ).
In subgroups of patients with STEMI and those with elective PCI, heparin was administered after blood was sampled from the femoral artery or aorta. There was no significant difference between levels of MPO in plasma from the femoral artery and aorta in patients with STEMI or in control patients with elective PCI when heparin was administered after blood was sampled from the aorta ( Figure 4 ). However, when heparin was administered after blood was sampled from the femoral artery, there was a marked and significant increase in the level of MPO in plasma from the aorta in patients with STEMI and controls with elective PCI (p <0.05). These results are consistent with previous studies showing that heparin mobilizes MPO from the endothelium.





