3 Evaluation of the Patient with Diastolic Dysfunction
Restrictive Cardiomyopathy
Background
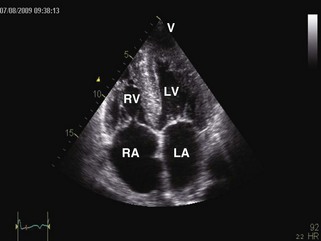
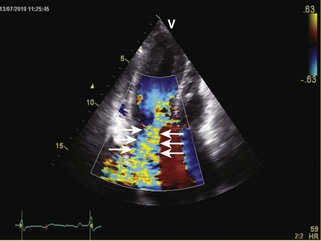
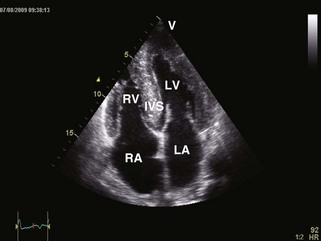
• Restrictive cardiomyopathies (RCMs) are rare disorders (Table 3-1) that cause abnormal stiffening of cardiac chambers, often associated with increased wall thickness (Figure 3-1). Contractile function is normal except in late stages of disease. Elevation of ventricular filling pressures and inability to augment stroke volume with exercise result in heart failure symptoms. Right heart failure often dominates the clinical picture.
• RCM and constrictive pericarditis (CP) have similar clinical presentations and echocardiographic findings. Differentiating RCM from CP is imperative as CP can be cured with pericardiectomy. The distinction is often difficult to make with echocardiography alone and requires integrating physiologic data from multiple investigations (Tables 3-2 and 3-3).
TABLE 3-1 CLASSIFICATION OF RCMs
| Site of Involvement | Classification |
|---|---|
| Myocardium | Noninfiltrative |
| Infiltrative | |
| Storage | |
| Endomyocardium |
TABLE 3-2 OVERALL ECHOCARDIOGRAPHIC APPROACH FOR ASSESSING DIASTOLIC DYSFUNCTION
| Modality | View/Technique | Findings |
|---|---|---|
| 2D | Apical, parasternal, subcostal | |
| Pulsed wave Doppler | 4-chamber: Ultrasound beam aimed at an angle of 20 degrees laterally to apex, and the sample volume between the tips of the mitral valve leaflets | |
| Suprasternal short-axis | ||
| Subcostal | ||
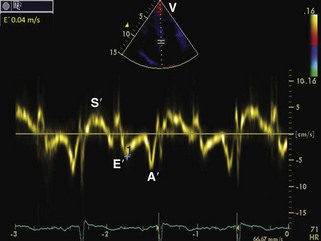
| Tissue Doppler imaging | 4-Chamber: 2-mm sample volume at the medial mitral annulus with the Doppler beam parallel to the longitudinal movement. Gain settings and wall filters should be low and velocity scale expanded. | Varying E, E/E patterns (see text for details) |
TABLE 3-3 Approaches and Findings in RCM
| Modality | General Findings | Specific Findings |
|---|---|---|
| Chest radiograph | Normal-sized heart. Dilated atria. Signs of pulmonary congestion or interstitial edema. Pleural effusion may occur. | Mediastinal lymphadenopathy; pulmonary parenchymal disease in sarcoidosis. |
| Electrocardiogram | Nonspecific ST- and T-wave abnormalities. Conduction abnormalities. Same chamber and wall dimensions and functions as in echocardiography. | Low voltage in precordial leads is seen in ~50% of patients with amyloidosis with cardiac involvement. Pseudo-infarct pattern (QS wave in consecutive leads) is seen in ~50% of patients with cardiac amyloidosis. Fibrosis caused by cardiac sarcoidosis can be detected with late gadolinium enhancement. Trilaminar appearance (normal myocardium, thickened fibrotic endocardium, and overlying thrombus) may be detectable in endomyocardial diseases. Global reduction in T2* cardiac tissue is commonly seen in hemochromatosis. |
| CT | Same chamber and wall dimensions and functions as in echocardiography. | |
| Biposy | In most cases nonspecific. | Apple-green birefringence, electron microscopy findings in amyloidosis. |
| Radionuclide imaging | Mainly nonspecific findings in RCM. | With increased cardiac involvement in amyloidosis, Tc-99m labeled tracers are detectable. |
| Cardiac catheterization | Nonspecific findings of diastolic dysfunction (increased diastolic pressures, right-sided square root sign, and often LVEDP ≥5 mm Hg greater than RVEDP). |
LVEDP, LV end-diastolic pressure; RVEDP, RV end-diastolic pressure; Tc-99m, 99-mtechnetium.
Echocardiographic Approach
General characteristic findings in RCM include (Figures 3-2 through 3-5):
• Abnormal LV diastolic function
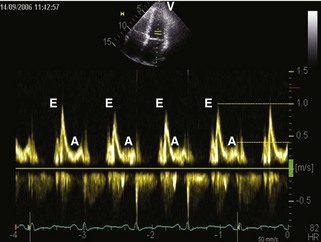
• Mitral inflow: mitral inflow early-to-late diastolic velocity ratio (E/A) greater than 1.5, deceleration time (DT) less than 150 ms, isovolumic relaxation time (IVRT) < 60 ms
Specific Diagnoses
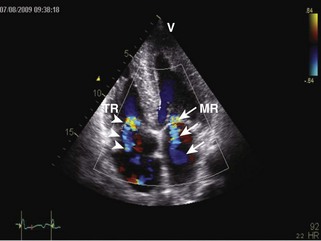
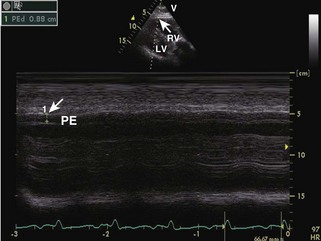
• Cardiac amyloidosis: increased RV/LV wall thickness, thickening of the interatrial septum and atrioventricular valves, presence of a small-to-moderate pericardial effusion, and “speckled” or “granular” myocardium (Figures 3-6 and 3-7).
• Cardiac sarcoidosis: Idiopathic, inflammatory multisystem granulomatous disease that commonly involves the lungs, skin, kidneys, eyes, and heart. Echocardiographic findings include both normal and dilated ventricular chambers; increased wall thickness and wall thinning, depending on stage and treatment, and aneurysm formation; segmental wall motion abnormalities not conforming to a coronary vascular distribution; and akinetic/dyskinetic wall segments adjacent to normokinetic wall segments.
• Glycogen storage disease: Unexplained left ventricular hypertrophy should prompt consideration of hypertrophic cardiomyopathy (HCM; see below) and the following glycogen storage disorders:
• Fabry’s disease: X-linked autosomal recessive disorder caused by lack of α-galactosidase A, leading to intracellular accumulation of ceramide trihexoside. The echocardiographic appearance is similar to HCM (see below). A specific finding for Fabry’s disease is a binary appearance of the LV endocardial border, reflecting the endocardial and subendocardial compartmentalization of glycosphingolipid material.
• Danon’s disease: X-linked disorder typically affecting young men (<20 years old) caused by deficient lysosomal-associated membrane protein 2 (LAMP2). Phenotype of marked concentric HCM (wall thickness 20 to 60 mm) with severe LV systolic dysfunction, often associated with ventricular preexcitation. Extracardiac clinical features include skeletal myopathy, mental retardation, and hepatic disease, but systemic features may be absent or minimal.
• PRKAG2 cardiomyopathy: Autosomal dominant mutation in regulatory subunit of AMP-activated receptor kinase. Electrocardiographic (ECG) findings include preexcitation. Differentiated from Danon’s disease clinically by absence of systemic disease.