Treatment of all coronary arteries is important to improve the prognosis of acute coronary syndrome after early reperfusion of the culprit lesion. Early statin treatment has been reported to cause regression of plaques away from the site of the culprit lesion in patients with acute coronary syndrome. However, the precise mechanism of coronary plaque regression is not well understood. We studied the effects of statins on the regression of coronary plaques away from the culprit lesions in 120 patients with acute coronary syndrome. We used virtual histology-intravascular ultrasound studies to evaluate nonpercutaneous coronary intervention lesions at admission and short-term (2 to 3 weeks) and medium-term (8 to 10 months) follow-up. According to the medium-term evaluation findings, the subjects were divided into 2 groups: a plaque regression group (n = 94) and a plaque progression group (n = 26). In the regression group, the fibrofatty component had decreased at the short-term (−20.0% vs baseline) and had decreased further at the medium-term (−26.7%) evaluations. The fibrous component had also decreased at the short-term (−5.1%) and medium-term (−8.5%) evaluations. In contrast, the necrotic core component showed a tendency to increase in the short term (+12.5%) but then decreased at the medium-term evaluation (−6.3%). In the progression group, the fibrofatty and fibrous components had increased at the short-term (+37.5%, +11.3%) and medium-term (+50.5%, +13.2%) evaluations; however, the necrotic core had decreased at the short-term (−19.0%) and medium-term (−23.8%) evaluations. In conclusion, regarding the course of coronary plaque regression by statin therapy, the plaques began to reduce the volume of fibrofatty and fibrous components in the early phase, associated with a transiently increased necrotic core component. Furthermore, even in the case of plaque progression, statins caused a reduction in the necrotic core.
To elucidate the process of plaque regression by statins, we observed the serial changes in the structure of nonculprit plaques in patients with acute coronary syndrome (ACS) using repeated virtual histology-intravascular ultrasound (VH-IVUS) studies.
Methods
The present study was designed as a prospective, nonrandomized, noncontrolled, and open-label trial. A total of 131 patients with ACS, who had undergone percutaneous coronary intervention (PCI), were eligible for enrollment in the present study. ACS was defined as unstable angina of Braunwald’s class IIIB (angina at rest without increased levels of the creatine kinase-MB fraction within 24 hours before coronary angiography), non–ST-segment elevation myocardial infarction, or ST-segment elevation myocardial infarction (n = 8, n = 13, and n = 110, respectively). Myocardial infarction was diagnosed by typical chest symptoms, electrocardiographic evidence of myocardial ischemia, and elevation of serum creatine-MB levels greater than twice the upper limit of normal. After emergent PCI for the culprit lesion, all the patients underwent a baseline VH-IVUS study for a nonculprit lesion, for which PCI had not been performed. Thereafter, all the patients were treated with either 10 mg/day atorvastatin (n = 66) or 2 mg/day pitavastatin (n = 65), the standard dose in Japan. The exclusion criteria were the absence of plaques suitable for VH-IVUS examination, failed PCI, a culprit lesion in the left main trunk, recommended coronary artery bypass grafting, cardiogenic shock, and renal or hepatic dysfunction. The patients who had already received lipid-lowering drugs, including statins, before enrollment were also excluded. VH-IVUS studies were performed at baseline and repeated on the same lesion at 2 to 3 weeks (short term) and 8 to 10 months (medium term) after the initiation of statin therapy. We compared the plaque composition at baseline and short- and medium-term follow-up using the VH-IVUS findings. Patient safety was assessed through adverse event reporting, physical examinations, electrocardiographic monitoring, and clinical laboratory tests. The Dokkyo Medical University ethics committee well recognized the importance of the present study and approved the study protocol. We carefully explained the study protocol, including the risks and benefits of the 3 VH-IVUS examinations to all the patients and their families, and all patients provided written informed consent.
The target lesion for intravascular ultrasonography was determined according to the following criteria: de novo and no significant stenosis (angiographic lumen diameter <50%) but with the presence of plaque; calcification that would not limit quantitative assessment of the cross-sectional area; an angiographic reference diameter of ≥3.0 mm and segment length of 5 to 15 mm; distance from the PCI site of ≥5.0 mm; and serial, high-quality IVUS of the entire segment.
The IVUS radio frequency (RF) data were acquired with a 20-MHz, 2.9F, phased-array IVUS catheter (Eagle Eye Gold, Volcano, Rancho Cordova, California) and a dedicated console (IVG3, Volcano). The image acquisition was electrocardiographically gated. The catheter probe was advanced ≥10 mm distal to the most distal side branch. Angiographic cine runs were performed to define the position of the IVUS catheter. Automated continuous pullback (0.5 mm/s) was performed after intracoronary administration of isosorbide dinitrate (2.5 mg). The IVUS RF data were stored on a hard disk for offline analysis. Manual contour detection of both the lumen and the media–adventitia interface was performed for the target segment. Quantitative and qualitative analysis of the target segments was performed with VH software (IVUS VH1.3j, Volcano). VH uses IVUS RF data to classify a plaque into 4 components: fibrous, fibrofatty, dense calcium, and necrotic core. These components are assigned corresponding color codes of green, greenish-yellow, white, and red, and color-coded tissue maps are constructed. The components can be identified within the plaque, as previously validated by studies in vitro and in vivo. The IVUS volumetric analysis was from gray scale IVUS images and included the external elastic membrane volume, lumen volume, plaque volume, and segment length. The plaque volume was calculated as the external elastic membrane volume minus the lumen volume (i.e., correctly, the plaque volume refers to a volume of plaque plus media). In addition, the volumes for the fibrous, fibrofatty, dense calcium, and necrotic core components were calculated by multiplying the mean of each component area of the region of interest by its length. Each component value was expressed in cubic millimeters. Each volume index (VI) was defined as the volume divided by the segment length. The change in each VI was calculated as (follow-up VI − baseline VI) and the percentage of change in the VI as [(change in VI/baseline VI) × 100]. Exact matching of the target site on the baseline and follow-up IVUS images was ensured by using side-by-side comparison of the serial IVUS image sequences, together with information of the pullback speed and characteristic calcifications, vascular and perivascular landmarks, side branches, and plaque shapes. IVUS analysis was performed once at baseline and at 2 follow-up points by the same experienced investigator (K.O.), who was unaware of the patient groups. The volumetric IVUS RF-based analysis using this method has shown acceptable reproducibility.
Statistical analysis was performed with StatView, version 5.0 (SAS Institute, Cary, North Carolina). Quantitative data are presented as the mean ± SD. The differences between 2 groups were assessed using a chi-square test for categorical variables and an unpaired Student t test for continuous variables. Differences in the continuous variables between baseline and follow-up were assessed using a paired Student t test. The percentage of changes from baseline to follow-up was tested using a 1-sample t test. A value of p <0.05 was considered statistically significant in all analyses.
Results
All 131 eligible patients underwent the short-term IVUS assessment; however, 11 patients were excluded for the medium-term assessment because of withdrawal of consent by 7, failure to follow-up by 1, and unacceptable IVUS images in 3 patients. No complications occurred from the IVUS procedures in any study patient. Using the medium-term IVUS findings, we determined whether the plaque had regressed or progressed. We defined plaque regression as the plaque VI at the medium-term examination that was less than or equal to the baseline value and plaque progression as the plaque VI at the medium-term examination that was greater than the baseline value. Thus, of the 120 patients, 94 (78.3%) showed plaque regression and 26 (21.7%) plaque progression.
The baseline characteristics of the patients are listed in Table 1 . No significant difference was seen in the baseline characteristics between the 2 groups of plaque regression and plaque progression. However, diabetes mellitus tended to be complicated more in the plaque progression group than in the plaque regression group (p = 0.09).
| All Patients (n = 120) | Plaque Regression (n = 94) | Plaque Progression (n = 26) | p Value | |
|---|---|---|---|---|
| Age (yrs) | 65.3 ± 9.8 | 65.8 ± 16.2 | 63.7 ± 16.5 | 0.48 |
| Men | 90 (75.0%) | 72 (76.6%) | 18 (69.2%) | 0.44 |
| Body mass index (kg/m 2 ) | 24.1 ± 2.6 | 24.0 ± 2.5 | 24.2 ± 2.7 | 0.30 |
| Hypertension | 73 (60.8%) | 59 (62.8%) | 14 (53.8%) | 0.41 |
| Dyslipidemia | 90 (75.0%) | 73 (77.7%) | 17 (65.4%) | 0.20 |
| Diabetes mellitus | 61 (50.1%) | 44 (46.8%) | 17 (65.4%) | 0.09 |
| Smoker | 41 (34.2%) | 33 (35.1%) | 8 (30.1%) | 0.68 |
| Atorvastatin/pitavastatin | 60/60 | 45/49 | 15/11 | 0.41 |
| Medications | ||||
| Angiotensin receptor blockers | 89 (74.2%) | 70 (74.5%) | 19 (73.1%) | 0.89 |
| Angiotensin-converting enzyme inhibitors | 26 (21.7%) | 20 (21.3%) | 6 (23.1%) | 0.84 |
| β Blockers | 47 (39.2%) | 38 (40.4%) | 9 (34.6%) | 0.59 |
| Calcium channel blockers | 61 (50.8%) | 44 (46.8%) | 17 (65.4%) | 0.11 |
| Target coronary artery for intravascular ultrasonography | 0.90 | |||
| Left anterior descending artery | 45 (37.5%) | 36 (38.3%) | 9 (34.6%) | |
| Left circumflex artery | 36 (30.0%) | 29 (30.9%) | 7 (26.9%) | |
| Right coronary artery | 21 (17.5%) | 16 (17.0%) | 5 (19.2%) | |
| Left main trunk | 18 (15.0%) | 13 (13.8%) | 5 (19.2%) | |
| Diseased vessels (n) | 0.72 | |||
| 1 | 99 (82.5%) | 80 (85.1%) | 19 (73.1%) | |
| 2 | 16 (13.3%) | 11 (11.7%) | 5 (19.2%) | |
| 3 | 5 (4.2%) | 3 (3.2%) | 2 (7.7%) | |
| Classification of acute coronary syndrome | 0.43 | |||
| Unstable angina | 6 (5.0%) | 4 (4.3%) | 2 (7.7%) | |
| Non–ST-segment elevation myocardial infarction | 10 (8.3%) | 7 (7.4%) | 3 (11.5%) | |
| ST-segment elevation myocardial infarction | 104 (94.5%) | 83 (88.3%) | 21 (80.8%) | |
| Medical history | ||||
| Myocardial infarction | 26 (21.7%) | 19 (20.2%) | 7 (26.9%) | 0.64 |
| Coronary revascularization | 31 (25.8%) | 22 (23.4%) | 9 (34.6%) | 0.25 |
| Follow-up interval (days) | ||||
| Short term | 18.1 ± 4.1 | 18.2 ± 4.2 | 17.7 ± 3.9 | 0.81 |
| Medium term | 269.1 ± 51.1 | 268.3 ± 52.9 | 272.0 ± 47.5 | 0.68 |
The lipid profile data, including the baseline values and the percentage of change from baseline at the short- and medium-term evaluations, are listed in Table 2 . No significant difference was seen in the baseline lipid profiles or the percentage of change at the short- and medium-term assessments between the 2 groups. However, the percentage of change in low-density lipoprotein cholesterol at the medium-term evaluation tended to be more prominent in the plaque regression group than in the plaque progression group (p = 0.09).
| All Patients (n = 120) | Plaque Regression (n = 94) | Plaque Progression (n = 26) | p Value | |
|---|---|---|---|---|
| Low-density lipoprotein cholesterol | ||||
| Baseline (mg/dl) | 117.1 ± 33.1 | 117.3 ± 34.7 | 116.2 ± 26.7 | 0.85 |
| Change at short-term (%) | −34.8 ± 18.0 ∗ | −35.7 ± 18.0 ∗ | −31.6 ± 18.3 ∗ | 0.31 |
| Change at medium-term (%) | −32.1 ± 17.8 ∗ | −33.3 ± 18.6 ∗ | −27.8 ± 13.7 ∗ | 0.09 |
| High-density lipoprotein cholesterol | ||||
| Baseline (mg/dl) | 46.7 ± 11.8 | 46.8 ± 10.9 | 46.5 ± 11.4 | 0.59 |
| Change at short-term (%) | −2.9 ± 2.5 | −2.7 ± 2.3 | −3.1 ± 2.8 | 0.22 |
| Change at medium-term (%) | −4.0 ± 3.1 | −4.3 ± 3.7 | −3.6 ± 2.6 | 0.45 |
| Triglycerides | ||||
| Baseline (mg/dl) | 117.6 ± 28.6 | 115.6 ± 22.6 | 119.9 ± 35.2 | 0.15 |
| Change at short-term (%) | −5.9 ± 4.4 | −5.6 ± 3.3 | −6.9 ± 4.8 | 0.62 |
| Change at medium-term (%) | −32.5 ± 20.9 ∗ | −31.5 ± 22.7 ∗ | −34.1 ± 18.9 ∗ | 0.39 |
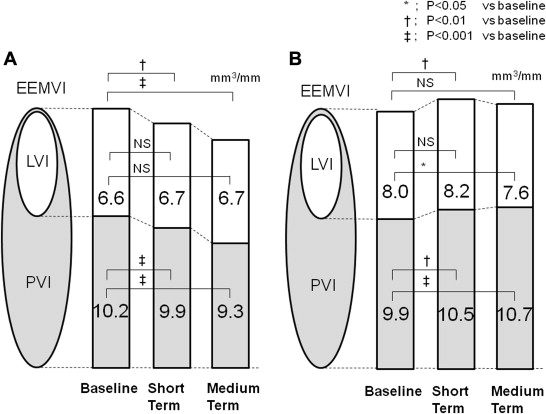
The average target lesion length in the plaque regression group and plaque progression group was 10.2 ± 4.8 and 10.4 ± 4.4 mm, respectively. Volumetric analysis of the plaques using gray scale IVUS images showed that the external elastic membrane VI, lumen VI, and plaque VI were similar between the 2 groups at baseline. In the plaque regression group, the plaque VI had begun to decrease at the short-term assessment (from 10.2 ± 3.0 to 9.9 ± 2.7 mm 3 /mm, −2.9%, p <0.001) and had decreased further at the medium-term evaluation (to 9.3 ± 2.5 mm 3 /mm, −8.8%, p <0.001 vs baseline). The external elastic membrane VI had also decreased at the short-term (16.8 ± 4.6 to 16.6 ± 4.5 mm 3 /mm, −1.1%, p <0.001) and medium-term (to 16.0 ± 4.5 mm 3 /mm, −4.7%, p <0.001 vs baseline) examinations. The lumen VI had not changed significantly at the short- or medium-term evaluation. In contrast, in the progression group, the plaque VI had begun to increase at the short-term evaluation (9.9 ± 2.9 to 10.5 ± 2.8 mm 3 /mm, +5.7%, p = 0.006) and had increased further at the medium-term evaluation (to 10.7 ± 3.1 mm 3 /mm, +7.5%, p <0.001 vs baseline). The external elastic membrane VI had also increased at the short-term assessment (17.9 ± 5.0 to 18.8 ± 4.5 mm 3 /mm, +5.2%, p = 0.002) but had then decreased to the baseline level at the medium-term evaluation (to 18.3 ± 4.8 mm 3 /mm). The lumen VI had not changed significantly at the short-term examination but had decreased to less than the baseline level at the medium-term examination (8.0 ± 2.8 to 7.6 ± 2.6 mm 3 /mm, −5.0%, p = 0.046 vs baseline; Figure 1 ).