The aim of this study was to assess the effects on lipids and safety during a 12-week reversal period after 18 months of treatment with anacetrapib. The cholesteryl ester transfer protein inhibitor anacetrapib was previously shown to reduce low-density lipoprotein cholesterol by 39.8% (estimated using the Friedewald equation) and increase high-density lipoprotein (HDL) cholesterol by 138.1%, with an acceptable side-effect profile, in patients with or at high risk for coronary heart disease in the Determining the Efficacy and Tolerability of CETP Inhibition With Anacetrapib (DEFINE) trial. A total of 1,398 patients entered the 12-week reversal-phase study, either after completion of the active-treatment phase or after early discontinuation of the study medication. In patients allocated to anacetrapib, placebo-adjusted mean percentage decreases from baseline were observed at 12 weeks off the study drug for Friedewald-calculated low-density lipoprotein cholesterol (18.6%), non-HDL cholesterol (17.6%), and apolipoprotein B (10.2%); placebo-adjusted mean percentage increases were observed for HDL cholesterol (73.0%) and apolipoprotein A-I (24.5%). Residual plasma anacetrapib levels (about 40% of on-treatment apparent steady-state trough levels) were also detected 12 weeks after cessation of anacetrapib. No clinically important elevations in liver enzymes, blood pressure, electrolytes, or adverse experiences were observed during the reversal phase. Preliminary data from a small cohort (n = 30) revealed the presence of low concentrations of anacetrapib in plasma 2.5 to 4 years after the last anacetrapib dose. In conclusion, after the cessation of active treatment, anacetrapib plasma lipid changes and drug levels decreased to approximately 40% of on-treatment trough levels at 12 weeks after dosing, but modest HDL cholesterol elevations and low drug concentrations were still detectable 2 to 4 years after the last dosing.
Multiple clinical trials with statins have demonstrated conclusively that reductions in low-density lipoprotein (LDL) cholesterol decrease the risk for cardiovascular events in patients with or without manifest coronary heart disease. In patients with existing disease, low levels of high-density lipoprotein (HDL) cholesterol is associated with increased risk even when LDL cholesterol is reduced to low levels with statin therapy. Inhibition of cholesteryl ester transfer protein is a strategy under investigation for increasing HDL cholesterol levels to reduce residual cardiovascular risk. The randomized, double-blind, placebo-controlled, phase 3 trial Determining the Efficacy and Tolerability of CETP Inhibition with Anacetrapib (DEFINE) evaluated the safety of the cholesteryl ester transfer protein inhibitor anacetrapib in patients with or at high risk for coronary heart disease. In the 76-week DEFINE study, treatment with anacetrapib was associated with a placebo-adjusted 39.8% reduction in Friedewald equation–calculated LDL cholesterol (the primary efficacy end point) and a 138.1% increase in HDL cholesterol in patients treated with statins with or without other lipid-modifying agents. A subsequent assay comparison study indicated that the Friedewald equation, the method used to calculate LDL cholesterol in DEFINE, underestimates LDL cholesterol levels after treatment with anacetrapib, compared with the reference β-quantification method. Treatment with anacetrapib for 76 weeks was well tolerated, with no clinically important differences in the predefined key safety parameters, including blood pressure, electrolytes, aldosterone, and prespecified cardiovascular adjudicated events. The present report describes lipid levels, drug levels, and safety parameters after cessation of anacetrapib in the DEFINE study. Because a previous phase 2b anacetrapib dose-ranging study had observed detectable anacetrapib drug levels with residual effects on LDL cholesterol and HDL cholesterol at 8 weeks after cessation of therapy, the present study involved a 12-week off-drug follow-up period.
Methods
Details of the design, objectives, and methods of DEFINE ( ClinicalTrials.gov identifier NCT00685776 ) have been previously reported. From April 1, 2008, to January 15, 2009, a total of 2,757 men and women aged 18 to 80 years with previous known coronary heart disease or at high risk for coronary heart disease (10-year Framingham risk score >20%) were screened from 153 centers in 20 countries. Of these, 1,697 entered the 2-week placebo run-in phase, and 1,623 were randomized into the trial. Eligible participants had LDL cholesterol levels of 50 to 100 mg/dl (by the Friedewald equation) while taking statins with or without other lipid-modifying medications, HDL cholesterol levels <60 mg/dl, and triglyceride levels <400 mg/dl. The study protocol was reviewed and approved by the institutional review board at each participating center.
During the active-treatment phase of the trial, patients were randomized to anacetrapib 100 mg or placebo for 76 weeks (weeks 0 to 76). During the reversal phase, patients, including those who discontinued the study medication early, were followed for a 12-week period to assess the residual effects on lipids, plasma levels of anacetrapib, and safety parameters.
In addition, a substudy was initiated in the United States (PN 019-05) to determine if anacetrapib levels could be detected in the plasma of patients who were treated for up to 77 weeks and were off the study drug for ≥2 years and up to 4 years. Preliminary data are available for 48 patients previously treated with either anacetrapib (n = 30) or placebo (n = 18) from 16 sites. These patients were invited for 1 clinic visit, at which blood samples were collected to assess plasma levels of anacetrapib and to determine nonfasting LDL cholesterol (direct Genzyme assay method; Genzyme, Cambridge, Massachusetts) and HDL cholesterol (dextran-sulfate method). Anacetrapib was not detected in the plasma of any of the placebo-treated subjects. Concomitant medication data were not collected from these patients.
No specific hypotheses were prespecified in this reversal study, so all inferential analyses and results are descriptive in nature. Measurements of blood lipids and biochemical safety parameters were obtained from blood samples from patients at 12 weeks after cessation of the study medication. Lipid end points during the reversal phase were the placebo-adjusted mean percentage change from baseline (week 0) in LDL cholesterol (by the Friedewald equation), HDL cholesterol, non-HDL cholesterol, apolipoprotein B, apolipoprotein A-I, and total cholesterol after completion of the 12-week reversal period. Safety variables included adverse experiences, blood pressure, serum electrolytes, and hepatic and muscle-related enzyme levels. The prespecified cardiovascular composite end point used to evaluate cardiovascular safety included death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, and hospitalization for unstable angina. All serious cardiovascular events and deaths from any cause were adjudicated by an expert committee blinded to treatment assignment and independent of the steering committee, site investigators, and study sponsor.
Blood samples were collected at visits at weeks 12, 24, and 76 during the treatment phase for determination of plasma anacetrapib concentration. The time of dosing anacetrapib on the day before the day of the visit was recorded only for the week 12 visit. Blood samples were also collected 12 weeks after the last dose of the study medication during the reversal phase for determination of plasma anacetrapib concentrations. In patients allocated to the anacetrapib arm, the geometric means of the available plasma anacetrapib concentrations at weeks 12, 24, and 76 during the treatment phase and at the 12-week reversal phase visit were calculated. On the basis of the plasma concentration profile of anacetrapib after multiple-dose studies (unpublished data), samples collected within 19 to 40 hours of the previous dose had plasma levels within 20% of the true trough concentration. Therefore, for the 12-week active-treatment phase visit, samples collected within 19 and 40 hours of the previous dose were defined as trough samples.
The primary efficacy analysis was based on a 12-week interval (i.e., end of study drug dosing to end of the 12-week reversal period). An analysis-of-covariance model was used for percentage change from baseline (week 0) at the 12-week reversal visit for LDL cholesterol, HDL cholesterol, non-HDL cholesterol, and total cholesterol; the analysis-of-covariance model included a term for treatment and used the respective lipid baseline term as a covariate. The treatment difference in terms of percentage change from baseline at the week 12 reversal visit was estimated from this model. Between-group estimates and 95% confidence intervals were provided for the anacetrapib versus placebo treatment groups. A sensitivity analysis was also performed to examine the effects on LDL cholesterol and HDL cholesterol and anacetrapib plasma levels for patients who discontinued early in the study and for patients who completed the 76 weeks of treatment. Key safety variables or adverse events of special interest were summarized for all patients who entered the reversal phase up to and including the 12-week reversal visit.
Results
Of the 1,623 patients randomized in the 76-week active-treatment phase of the DEFINE study, a total of 1,398 entered the reversal-phase study after cessation of the study drug. Two types of patients entered the 12-week reversal phase: (1) those who previously completed the 76-week treatment phase and (2) those who discontinued early ( Figure 1 ). Baseline patient characteristics were similar between the active treatment and reversal phases ( Table 1 ).

| Characteristic | Active Treatment (n = 1,623) | Reversal (n = 1,398) |
|---|---|---|
| Age (yrs) | 62.7 ± 8.9 | 62.6 ± 8.8 |
| Men | 1,247 (76.8%) | 1,084 (77.5%) |
| Hypertension ∗ | 1,101 (67.8%) | 951 (68.0%) |
| Diabetes mellitus | 862 (53.1%) | 723 (53.2%) |
| Previous myocardial infarction † | 370 (22.8%) | 329 (23.5%) |
| Body mass index (kg/m 2 ) | 30.2 ± 5.3 | 30.0 ± 5.2 |
| Friedewald equation–calculated LDL cholesterol (mg/dl) | 81.8 ± 21.0 | 82.4 ± 20.6 |
| HDL cholesterol (mg/dl) | 40.4 ± 9.2 | 40.5 ± 9.1 |
∗ Terms included for hypertension: “blood pressure increased,” “essential hypertension,” “hypertension,” and “labile hypertension.”
† Terms included for myocardial infarction: “acute myocardial infarction” and “myocardial infarction.”
Evidence of a drug effect on lipid end points was observed after completion of the 12-week reversal period in patients allocated to the anacetrapib arm. At 12 weeks after cessation of anacetrapib, placebo-adjusted mean percentage reductions from baseline (week 0) were observed for LDL cholesterol (18.6%) estimated by the Friedewald equation, apolipoprotein B (10.2%), and non-HDL cholesterol (17.6%) ( Figure 2 ). Mean percentage increases in HDL cholesterol (73.0%) and apolipoprotein A-I (24.5%) were also evident ( Figure 2 ). These placebo-adjusted treatment effects on lipids after the reversal period were approximately 50% to 60% of those observed during the active-treatment phase. In patients who discontinued treatment early (n = 136), who had a median duration of treatment of 170 days, the effects on LDL cholesterol and HDL cholesterol 12 weeks after stopping therapy were smaller than in the patients who completed the 76-week treatment phase of therapy ( Table 2 ).

| Variable | Placebo-Adjusted Percentage Change from Baseline (95% Confidence Interval) | |
|---|---|---|
| LDL Cholesterol | HDL Cholesterol | |
| All patients in reversal ∗ | −18.6 (−21.5 to −15.7) | 73.0 (69.4 to 76.6) |
| n (anacetrapib/placebo) | 641/681 | 655/685 |
| Patients who completed 76 weeks of treatment ∗ | −20.1 (−23.0 to −17.2) | 79.3 (75.6 to 82.9) |
| n (anacetrapib/placebo) | 519/635 | 530/639 |
| Patients who discontinued during the treatment phase † | −11.0 (−21.5 to −0.7) | 39.6 (28.1 to 52.4) |
| n (anacetrapib/placebo) | 122/46 | 125/46 |
∗ Difference in least squares means estimated from the analysis-of-covariance model: percentage change from baseline at week 88 = treatment + baseline.
† Difference in median Hodges-Lehmann estimate of the difference between treatments with a corresponding distribution-free confidence interval based on Wilcoxon’s rank-sum test.
Anacetrapib plasma concentrations were relatively constant at weeks 12, 24, and 76 of the treatment phase ( Table 3 ). The geometric mean trough concentration at week 12 was 486 nmol/L in the subset of 298 patients from the treatment phase with available trough measurements. At 12 weeks after cessation of anacetrapib, the geometric mean anacetrapib concentration for all patients from the reversal phase was approximately 60% lower than the on-treatment geometric mean trough level. In the subset of 239 patients from the reversal phase with available trough concentrations at week 12 of the treatment phase, those who completed the 76-week treatment had a geometric mean anacetrapib concentration at the end of the reversal period that was 55% lower than the geometric mean trough concentration at the 12-week on-treatment time point; for those who discontinued treatment early, this level was 79% lower ( Table 4 ).
| Treatment | Treatment Phase | Reversal Phase (12 Weeks After Cessation of Study Drug) | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Week 12 (nmol/L) | n | Week 24 (nmol/L) | n | Week 76 (nmol/L) | n | Week 12 (nmol/L) | |
| All available data | 722 | 631 (73%) | 575 | 634 (77%) | 469 | 665 (84%) | 628 | 201 (66%) |
| Trough ∗ concentration | 298 | 486 (70%) | Not available | Not available | Not applicable | |||
∗ Pharmacokinetic samples obtained within 19.4 to 40 hours of the last dose.
| Group | n | Treatment Phase | Reversal Phase | Reversal/Treatment Week 12 Trough Ratio |
|---|---|---|---|---|
| Week 12 Trough (nmol/L) | Week 12 Concentration (nmol/L) | |||
| Patients who completed 76 weeks of treatment | 198 | 444 (58%) | 201 (43%) | 0.45 |
| Patients who discontinued during the treatment phase | 41 | 635 (62%) | 136 (61%) | 0.21 |
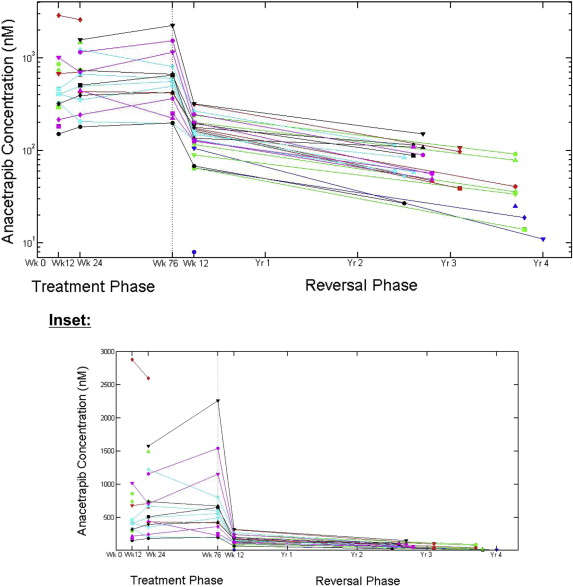
Anacetrapib levels were also assessed in 30 patients who were previously treated with anacetrapib for time periods ranging from 1 to 77 weeks but who did not enter the 2-year extension study ( ClinicalTrials.gov identifier NCT00685776 ). These patients were off the study drug for 2.4 to 4 years. Residual anacetrapib levels were still present at levels that are expected to indicate low pharmacologic activity ( Figure 3 ). The median percentage change in HDL cholesterol was an 18.1% residual increase from baseline in patients who were off treatment with anacetrapib for up to 4 years, compared with a 122.5% increase at the end of the treatment phase. However, this estimate needs to be interpreted cautiously because concomitant therapy information is not currently available for these patients.
During the 12-week reversal phase of the study, there were no patterns of clinically important differences in liver enzymes, blood pressure, electrolytes, or adverse experiences between the anacetrapib group and the placebo group ( Table 5 ). In 4 of the 5 measures of blood pressure, there were no significant differences between the 2 groups; however, there was a significant difference in the percentage of patients with increases in diastolic blood pressure of ≥10 mm Hg ( Table 5 ). A total of 97 patients (14.6%) in the anacetrapib group experienced elevations in diastolic blood pressure of ≥10 mm Hg during the reversal phase, compared with 74 (10.7%) in the placebo group (p = 0.028). Still, there were no significant differences between treatment groups in the mean change from baseline (week 0) for systolic or diastolic blood pressure or in the percentage of subjects with increases of ≥10 or ≥15 mm Hg in systolic blood pressure ( Table 5 , Supplementary Figure 1 ). There were also no significant between-group differences in serum levels of chloride, bicarbonate, or potassium ( Table 5 ). With regard to serum levels of sodium, there was no significant difference between treatment groups in the percentage of patients with values greater than the upper limit of the normal range at the 12-week reversal visit. However, the mean changes in sodium levels from baseline to the 12-week reversal visit were −1.1 and −1.5 mmol/L in the anacetrapib and placebo groups, respectively (p = 0.006).



