Fig. 12.1
Tetralogy of Fallot. Mid esophageal four chamber view showing right ventricular hypertrophy, the large malalignment ventricular septal defect (asterisk), and overriding aorta. LA left atrium, LV left ventricle, RV right ventricle
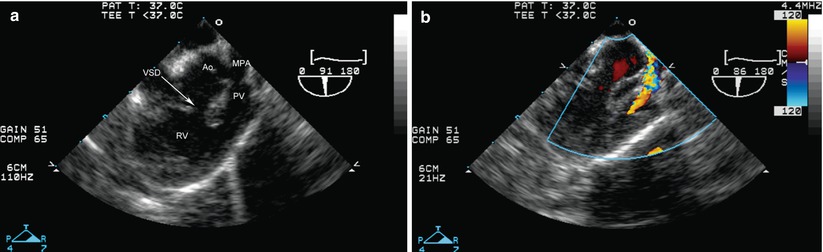
Fig. 12.2
Tetralogy of Fallot. (a) Modified mid esophageal right ventricular inflow-outflow view (multiplane angle about 90°) showing the malalignment ventricular septal defect (VSD), as well as narrowing of the right ventricular outflow due to a malaligned conal septum. (b) Color flow Doppler shows aliasing beginning at subpulmonary level, and continuing across the hypoplastic pulmonary valve. Ao aorta, MPA main pulmonary artery, PV pulmonary valve, RV right ventricle
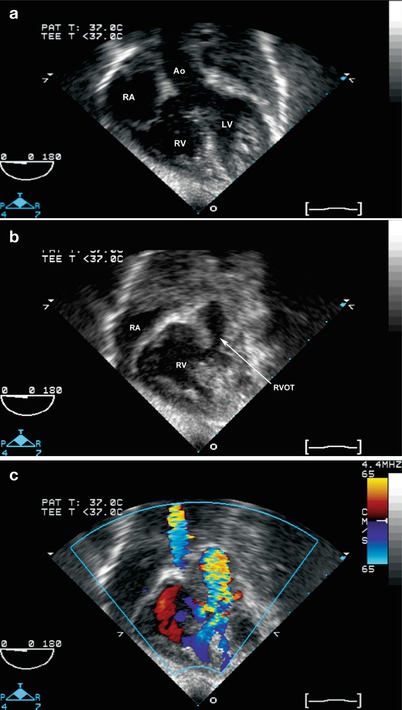
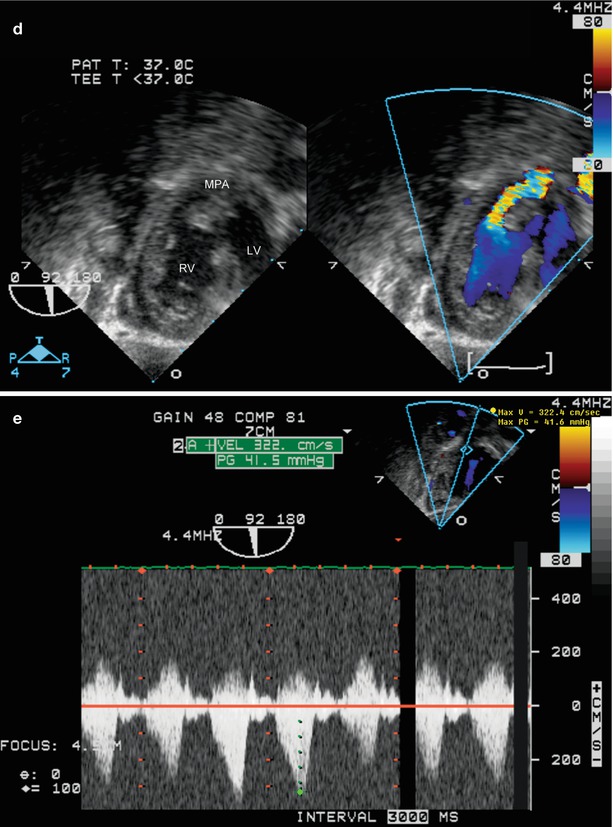
Fig. 12.3
Tetralogy of Fallot: deep transgastric long axis (a–c) and sagittal views (d, e) that simulate transthoracic subcostal coronal and sagittal views. (a–c) Shows the overriding aorta and malalignment ventricular septal defect (a). With slight probe withdrawal, the anteriorly malaligned infundibular septum is seen (b), producing subpulmonary stenosis, with color flow aliasing at this level (c). (d, e) Also show the anteriorly malaligned infundibular septum with color flow Doppler aliasing, as well as the ventricular septal defect with right to left shunting. The spectral Doppler tracing shows the typical “dagger” shape seen with subpulmonary stenosis. Ao aorta, LV left ventricle, MPA pulmonary artery, RA right atrium, RV right ventricle, RVOT right ventricular outflow tract
Color Doppler evaluation prior to surgical intervention includes assessment of flow across the atrial septum (typical flow pattern is from the left atrium to the right atrium). The ventricular septum can be interrogated in all views to assess for additional ventricular communications in the muscular septum. Spectral Doppler assessment of the gradient across the RV outflow tract is best performed in the mid esophageal ascending aortic short axis (ME Asc Ao SAX), ME RV In-Out or DTG LAX and Sagittal views—whichever yields a more favorable angle for Doppler evaluation (Figs. 12.2 and 12.3, Videos 12.2 and 12.3). To evaluate flow in the branch pulmonary arteries, and to search for a possible patent ductus arteriosus, color Doppler can be performed using the upper esophageal pulmonary artery long axis (UE PA LAX) and upper esophageal aortic arch short and long axis (UE Ao Arch SAX, UE Ao Arch LAX) views. It should be remembered that a significant proportion of TOF patients with a right-sided aortic arch (which occurs in about 25 % of TOF) will have a left sided ductus arteriosus originating from the left subclavian or innominate artery, and entering the main/left pulmonary artery [18].
Surgical Considerations
The operative repair of tetralogy of Fallot involves closure of the ventricular septal defect (Fig. 12.4, Video 12.4) and relief of the RV outflow tract obstruction. The outflow tract surgery is dependent upon the severity of infundibular and pulmonary valvar hypoplasia. A transannular outflow tract patch is used when the outflow tract obstruction is severe, with significant pulmonary valve annular hypoplasia. In milder forms of TOF, patch enlargement of the hypoplastic infundibulum (a non transannular patch), if required, is performed while the pulmonary valve is preserved to avoid long-term severe pulmonary regurgitation. In some centers, a transatrial approach is employed, in which the VSD is closed and RV muscle is resected from the right atrium, thereby minimizing or avoiding a right ventriculotomy [19]. Conduits from the RV to the pulmonary artery are required in many cases of pulmonary atresia or when a coronary (typically a left anterior descending from the right coronary) crosses the RV outflow tract. The coronary arteries can be evaluated preoperatively using the mid esophageal aortic valve short axis (ME AV SAX) view, as discussed in Chap. 4. With probe anteflexion and careful evaluation, it is possible to visualize a coronary crossing the RV outflow tract (Fig. 12.5, Video 12.5).
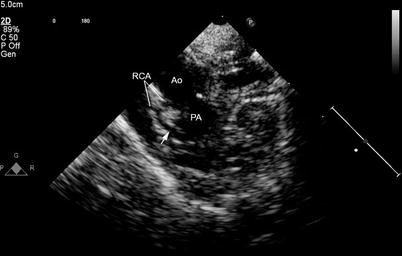

Fig. 12.4
Mid esophageal four chamber view demonstrating right ventricular hypertrophy in a patient with tetralogy of Fallot. Also note the presence of a ventricular septal defect patch (arrow), in good position

Fig. 12.5
Preoperative study in a patient with tetralogy of Fallot. A prominent anterior descending coronary artery (arrow) arises from the right coronary artery (RCA) and courses anterior to the right ventricular outflow tract, thereby precluding a transannular patch. Ao aorta, PA main pulmonary artery
Postoperative TEE is indicated to evaluate for (1) residual RV outflow tract obstruction, (2) residual VSDs (Figs. 12.6 and 12.7, Video 12.6), (3) assessment of ventricular performance, (4) severity of pulmonary regurgitation, and (5) flow direction across the atrial septum (if an intentional small communication is present). Branch pulmonary artery stenosis can be difficult to diagnose using TEE [20], but in some instances useful information about the branch pulmonary arteries can be obtained using the mid and upper esophageal views, using the techniques and views discussed in Chap. 4 (Fig. 12.8, Video 12.7). As in all forms of conotruncal malformations, all TEE views should be used to assess for residual VSD. The most common type of residual defect is a peri-patch leak between sutures (Figs. 12.6 and 12.7). Defects less than 3 mm tend to have minimal hemodynamic impact, though some defects of that size can be a concern, particularly in very small infants [21]. Determination of RV pressure either by Doppler interrogation of the tricuspid regurgitation jet (if present) or by the residual VSD jet, particularly when the RV outflow tract is unobstructed, is of utmost importance in assessment of hemodynamic significance of the residual VSD. By definition, a restrictive VSD is associated with RV pressure that is subsystemic. If RV pressure cannot be estimated by TEE, an assessment of pulmonary to systemic blood flow ratio (Qp/Qs), in which the surgeon directly obtains blood samples from the SVC and pulmonary and systemic arteries for oximetry, is helpful to determine the significance of the residual VSD.
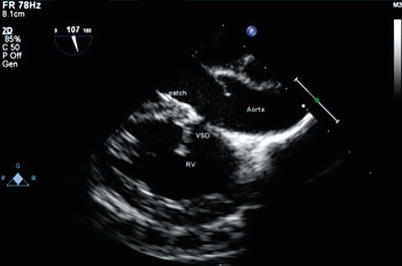
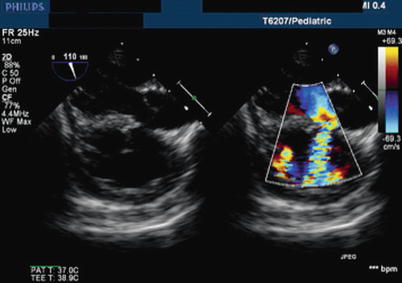
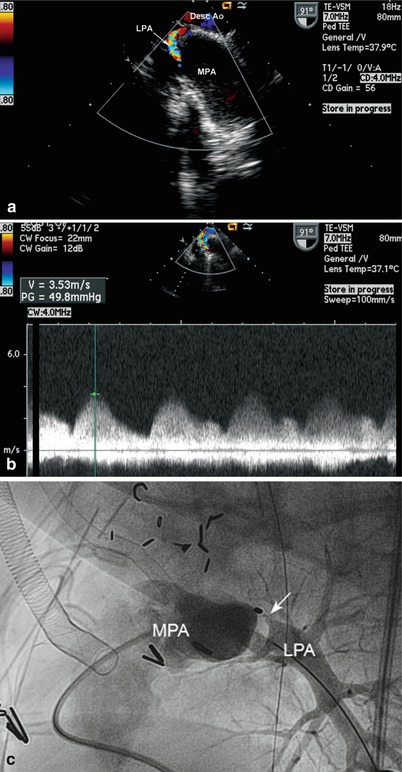

Fig. 12.6
Modified mid esophageal long axis view, with multiplane angle 107° in a patient with tetralogy of Fallot demonstrating a residual ventricular septal defect (VSD). The defect is located in the superior aspect of the VSD patch just under the aortic valve. RV right ventricle

Fig. 12.7
Modified mid esophageal long axis view, with multiplane angle 110°. This color compare image illustrates the presence of a residual ventricular septal defect after repair of tetralogy of Fallot; the defect is located immediately inferior to the aorta. The etiology of the defect is attributed to patch dehiscence. Here, the ventricular shunt direction is left to right

Fig. 12.8
(a) Upper esophageal aortic arch short axis with counterclockwise rotation, demonstrating significant left pulmonary artery stenosis in this patient with tetralogy of Fallot who underwent complete correction, including unifocalization of discontinuous pulmonary arteries. (b) Spectral continuous wave Doppler tracing showing the significant gradient across the stenotic area. (c) Postoperative pulmonary artery angiogram confirming the marked stenosis (arrow) of the proximal left pulmonary artery. Desc Ao descending aorta, LPA left pulmonary artery, MPA main pulmonary artery
One of the most challenging types of residual VSDs to assess is the intramural defect. This type of defect occurs in the setting of conotruncal malformations, when the VSD patch attaches to the trabeculated right ventricular free wall related to the ventriculoinfundibular fold (parietal band), creating a communication through the intertrabeculated spaces to the right ventricular cavity [21–23]. This area of the heart is difficult for the surgeon to visualize during the repair. Importantly, these defects can increase in size over time. The ME 4 Ch view highlights this type of residual defect well but it can also be seen with a modified ME RV In-Out view, in which the multiplane angle has been rotated to about 90°. Intramural residual VSDs should be looked for in all patients undergoing repair of conotruncal anomalies that include VSD closure.
Assessment of the RV outflow tract for residual obstruction is best performed in a modified ME RV In-Out view with rotation of the multiplane angle to 90–110° (Figs. 12.9 and 12.10, Video 12.8). Doppler interrogation of the RV outflow tract can be performed with this view, and withdrawal of the probe enables evaluation of the more distal portion of the outflow tract. In some cases where the outflow obstruction is more inferior, the DTG LAX and DTG Sagittal views can be used for optimal Doppler evaluation. A peak Doppler velocity that exceeds 3 m/s (36 mmHg) is considered potentially significant [24]. However, it should be noted that a hypercontractile state exists immediately following TOF repair, due in part to inotropic support and hypovolemia, which can accentuate a dynamic RV outflow tract gradient. Indeed, a study by Kaushal et al. evaluating 166 TOF patients immediately following transatrial repair (median age 7 years) found that 35 % had “significant” residual obstruction (gradient >40 mmHg, right:left ventricular pressure ratio >85 %). Most of these patients (88 %) had a dynamic, rather than fixed, obstruction as defined by TEE; patients with fixed obstruction underwent immediate surgical revision, while those with dynamic obstruction did not. Operative mortality and morbidity were not related to higher residual gradients, and on follow-up (mean 18.5 months) outflow gradients had declined sharply (mean 16 mmHg) irrespective of the severity of intraoperative gradients [25]. Thus a “significant” gradient in the immediate postoperative study should be viewed carefully; the nature of the obstruction (fixed vs. dynamic), and other intraoperative factors, should be considered when deciding to reoperate.
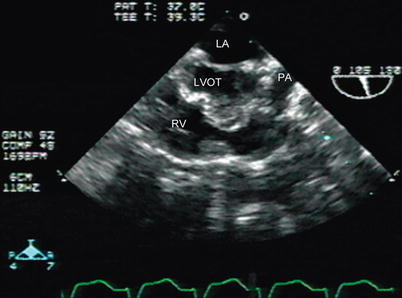
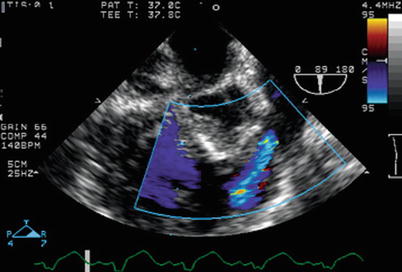

Fig. 12.9
Residual right ventricular outflow tract narrowing is seen in a patient after tetralogy of Fallot repair in a modified mid esophageal right ventricular inflow-outflow view, with slight probe withdrawal to display the outflow tract more clearly LA left atrium, LVOT left ventricular outflow tract, PA pulmonary artery, RV right ventricle

Fig. 12.10
Residual right ventricular outflow tract obstruction seen following tetralogy of Fallot surgery, with color Doppler interrogation in a modifed mid esophageal right ventricular inflow-outflow view. Following surgery, increased velocity in the right ventricular outflow tract is present (manifested by color flow Doppler aliasing), mostly related to dynamic subpulmonary obstruction
In the immediate postoperative hemodynamic assessment of the TOF patient, several other factors should be evaluated. Detection of intracardiac air and monitoring of cardiac de-airing can be performed using the ME 4 Ch and ME LAX views. Evaluation of right and left ventricular function can be performed with a number of mid esophageal and transgastric TEE views, including ME 4 Ch, mid esophageal two chamber (ME 2 Ch), ME RV In-Out, and the transgastric basal and mid short axis (TG Basal SAX, TG Mid SAX) views. The LV outflow tract is well visualized in both the ME AV LAX, multiplane angle 120–135° (Fig. 12.11, Video 12.9), and ME 4 Ch view (Fig. 12.12, Video 12.10) but this is rarely obstructed after TOF repair. Assessment of severity of pulmonary regurgitation can also be performed using the same views used to evaluate the RV outflow tract, including the ME RV In-Out view and the DTG Sagittal and LAX views. Some degree of pulmonary regurgitation can be expected after TOF repair, particularly following transannular patch. Several methods can be used to evaluate the degree of pulmonary regurgitation, including width of the color Doppler jet compared to outflow tract diameter (mild ≤1/3, moderate 1/3 to 2/3, severe ≥2/3) and presence of diastolic flow reversal in the branch pulmonary arteries [26]. However, changing hemodynamic and ventilatory conditions following TOF surgery can alter the amount of pulmonary regurgitation noted in the immediate postoperative period, compared to later in the postoperative course.
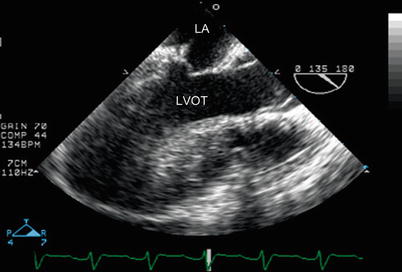
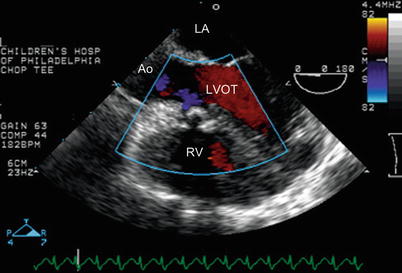

Fig. 12.11
Mid esophageal aortic valve long axis view demonstrating an unobstructed left ventricular outflow tract (LVOT) after repair of tetralogy of Fallot. The aortic valve is in the open position. LA left atrium

Fig. 12.12
Mid esophageal four chamber view with probe withdrawn to image the left ventricular outflow tract (LVOT) demonstrates that there is no residual VSD by color Doppler interrogation after tetralogy of Fallot repair. In addition, flow is laminar into the left ventricular outflow tract. Ao aorta, LA left atrium, RV right ventricle
In some TOF patients, a small atrial communication (usually a patent foramen ovale) is left patent as a “pop off” to facilitate cardiac output in the immediate postoperative period. The Doppler flow pattern across the foramen ovale is typically from right atrium to left atrium after surgery as a result of poor RV compliance (from the right ventriculotomy and RV hypertrophy) (Fig. 12.13). The atrial septum can be evaluated by sweeping from the inferior vena cava (IVC) to the superior vena cava. This sweep is performed by starting with the lower esophageal situs short axis (LE Situs SAX) view to image the IVC and hepatic veins, then slowly withdrawing the probe to the ME 4 Ch view (with slight rightward probe rotation) to visualize the atria, atrial septum and superior vena cava, while maintaining the multiplane angle between 0 and 30°.
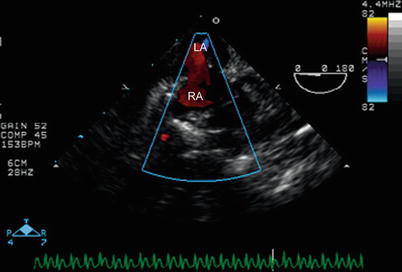

Fig. 12.13
Mid esophageal four chamber view, with probe withdrawn superiorly to the level of the atrial septum and rotated clockwise toward the patient’s right side. Color Doppler interrogation demonstrates a residual right to left shunt (red) through an atrial communication that was intentionally left patent during surgery. This type of shunting typically occurs in the early postoperative period after tetralogy of Fallot repair. LA left atrium, RA right atrium
In the case of a patient with TOF and AV canal defect, both atrial and ventricular septal patches should be evaluated for residual shunts. As with all AV canal repairs (Chap. 8), both AV valves (particularly the mitral valve) must be evaluated for possible regurgitation (Fig. 12.14, Video 12.11). However in addition, the RV outflow tract must be evaluated in the same manner as other TOF patients
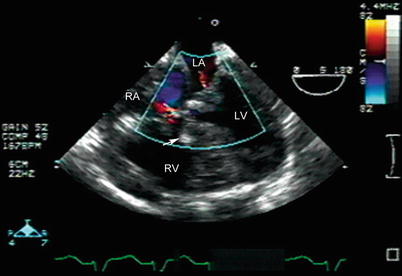

Fig. 12.14
Mid esophageal four chamber view in a patient who has undergone tetralogy of Fallot and atrioventricular canal repair. A large ventricular septal defect patch is seen (arrow). Minimal residual atrioventricular valve regurgitation is seen across the right and left atrioventricular valves. LA left atrium, LV left ventricle, RA right atrium, RV right ventricle
In older patients, TEE can be used to assess TOF repair including adults with poor acoustic windows. The long-term impact of severe pulmonary regurgitation can be gauged by evaluation of RV size and performance. When there appears to be significant negative impact of the regurgitation (manifested by marked RV dilation and/or deteriorating RV systolic function), prosthetic pulmonary valve implantation is indicated. This is usually performed surgically but in some instances, a transcatheter approach (notably using the Melody valve) can be employed (Chap. 16). In addition, tricuspid valve replacement might be indicated in cases of severe tricuspid regurgitation (Fig. 12.15, Video 12.12). RV systolic pressure estimate can be performed if tricuspid regurgitation is present. Aortic root dilation is a common finding in adults with TOF and can be associated with the development of aortic regurgitation over time. Patients with TOF can also develop important arrhythmias such as ventricular tachycardia. In some cases, TEE can help distinguish the type of arrhythmia.
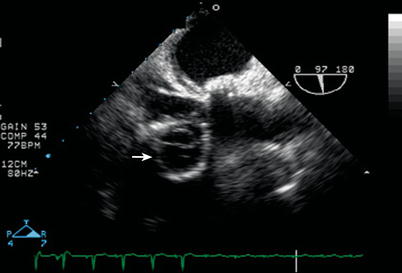

Fig. 12.15
Severe tricuspid regurgitation prompted tricuspid valve replacement in this adult long after tetralogy of Fallot repair; as seen here in a modified mid esophageal right ventricular inflow-outflow view (multiplane angle 97°), the prosthetic valve appears in cross section as indicated by the arrow
Double Outlet Right Ventricle
Anatomy
Double outlet right ventricle (DORV) is not a specific congenital malformation, but rather, an abnormal ventriculo-arterial alignment whereby both great arteries arise from the morphologic RV (Figs. 12.16 and 12.17, Video 12.13). By most criteria, both great vessels must sit more than 50 % over the RV [27]. Typically, there is bilateral conus, with myocardium separating both great vessels from the AV valves. However, other conal anatomy can occur in DORV [28]. A VSD is present in almost all cases and usually acts as the “outlet” from the left ventricle (LV). There are rare reports of DORV with an intact ventricular septum [29, 30]. In DORV, the ventricular loop may be “D” (normal) or “L” (inverted) and this will affect the relationship of the great arteries to the ventricle and to each other.
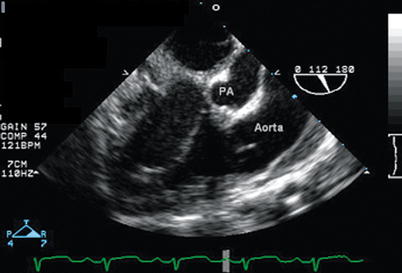
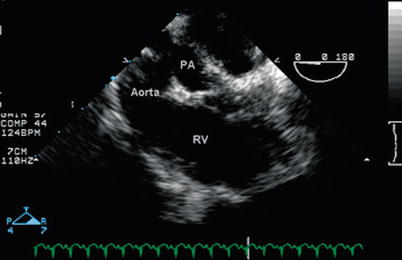

Fig. 12.16
Modified mid esophageal right ventricular inflow-outflow view (multiplane angle 110°) in a patient with double outlet right ventricle with posterior malalignment of the conal septum and subaortic ventricular septal defect, demonstrating that the aorta is anterior to the smaller pulmonary artery (PA). Both great vessels arise from the anterior right ventricle. The conal septum is seen between the aorta and PA

Fig. 12.17
Mid esophageal four chamber view with the probe withdrawn towards the base of the heart in the same patient displayed in Fig. 12.16 with double outlet right ventricle, posterior malalignment of the conal septum with subaortic ventricular septal defect; in this image, the aorta and pulmonary artery (PA) come into view, both arising from the right ventricle (RV). The PA is smaller than the aorta because of the conal septal malalignment
The anatomic and physiologic spectrum of DORV is wide. Associated anomalies are common and include ventricular hypoplasia, superior/inferior ventricles, mitral valve anomalies (including mitral atresia, straddling or parachute mitral valve), common AV canal (in heterotaxy syndrome) and outflow obstruction [28, 31, 32].
The pathophysiology can be similar to TOF, transposition of the great arteries, or a large VSD. This physiology is determined by the location of the great vessels in relationship to the VSD and the degree of outflow obstruction. Of critical importance is the type and location of the VSD: it often determines the type of surgical repair as well as, in some instances, the viability for two-ventricle versus a single ventricle surgical strategy. By far, the most common type of VSD in DORV is the malalignment type, however any type of VSD (such as AV canal, mid muscular, or apical muscular) can occur [33]. The VSD is also classified as one of four types, based upon its relationship to the great vessels: subaortic, subpulmonary, noncommitted, and doubly committed. In those patients with DORV with malalignment type VSD, the most common location of the VSD is subaortic. This is seen on echocardiography as muscle between the mitral and aortic valves. If there is no pulmonary stenosis, the physiology is similar to a large unrestrictive VSD because the blood flow streams from the LV into the aorta. If there is pulmonary stenosis, the physiology is similar to TOF. However, the distinction between DORV and TOF is of utmost importance because those patients with TOF-like DORV have complete subaortic conus that can result in subaortic obstruction after surgery [34]. Subaortic stenosis is extremely rare in TOF.
DORV with subpulmonary VSD is classically termed a “Taussig-Bing” anomaly. In this defect, the great vessels are spatially oriented side-by-side, bilateral conus is present, and the aorta is to the right of the pulmonary artery. The blood from the LV streams into the pulmonary artery so that the physiology is similar to transposition of the great arteries (see below). The subaortic conus is usually small, thus arch obstruction is a frequent association. Some cases of DORV with subpulmonary VSD are not classic Taussig-Bing anomalies but rather fall within the spectrum of that anatomic description. The distinction is critically important when determining surgical strategy.
More uncommon locations of the VSD in DORV include non-committed or doubly-committed subtypes. In DORV with non-committed VSD, the VSD is usually a muscular or a canal (inlet) type VSD. Since it is far from both outflow tracts, baffling procedures may pose a significant challenge. Often, AV valve tissue is obstructing the potential pathway from the LV to the aorta. Moreover, straddling tricuspid valve can occur in association with a canal type VSD. When a muscular VSD is present, there is a tendency for the defect to get smaller over time because it is surrounded by muscle; therefore in general, muscular VSDs are not considered reliable pathways to a great artery. In the rare doubly-committed type, both great vessels sit directly over the VSD.
Other variations of DORV exist, most commonly including those with mitral atresia or hypoplasia associated with left ventricular hypoplasia. When there is significant ventricular hypoplasia, the single ventricle surgical strategy is usually indicated. DORV is often seen in patients with superior/inferior ventricles with criss-cross AV valves. In this rare anomaly, the RV is situated superior to the LV and it is typically hypoplastic. The VSD is usually a canal (inlet) type.
Genetic syndromes can be seen with DORV but there is not one particular association. Transforming growth factor (TGF)-beta 2 knockout mice have been observed to develop DORV in the majority of cases [35]. Heterotaxy syndrome often has DORV as one of its cardiac components.
TEE Evaluation
There are pertinent findings seen with DORV to keep in mind when performing a TEE:
Location and size of the VSD.
Relationship of great vessels to the VSD.
Presence of additional VSDs.
Restrictive VSD due to accessory AV valve tissue.
Anatomy and size of AV valves.
Presence and severity of outflow tract obstruction (and to which great vessel).
Coronary anomalies, particularly if the arterial switch operation is being considered.
One of the most important aspects of the pre-operative evaluation of the patient with DORV is assessment of location and size of the VSD and its relationship to the great vessels (Fig. 12.18, Video 12.14). This, along with the other echocardiographic goals mentioned above, can often help in the determination of the optimal surgical strategy. TEE is an excellent tool to assess for AV valve abnormalities that might preclude a biventricular repair. For example, a straddling AV valve over a VSD usually obstructs the pathway from the LV to a great artery. TEE can also assess whether a VSD is restrictive and the mechanism of the restriction (i.e. AV valve tissue, conal muscle) [36]. Moreover, important anatomic features to help determine a surgical strategy include prominence of the conal septum, presence of tricuspid valve attachments to conal septum, and the distance between the pulmonary and tricuspid valves [37].
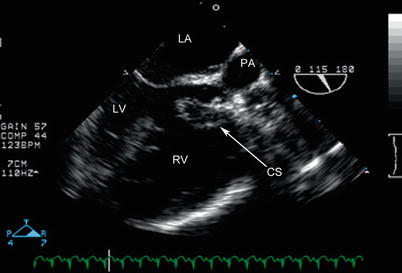

Fig. 12.18
Mid esophageal aortic valve long axis view in the same patient as Figs. 12.16 and 12.17 with double outlet right ventricle, posterior malalignment of the conal septum (CS), and subpulmonary ventricular septal defect, highlighting the posterior deviation of the conal septum that results in severe subpulmonary stenosis. The conal septum is also hypertrophied. LA left atrium, LV left ventricle, PA pulmonary artery, RV right ventricle
The standard ME 4 Ch view may not show evidence of pathology other than RV hypertrophy. However, probe withdrawal and anteflexion will demonstrate that both great vessels arise mostly or completely from the anatomic RV (Fig. 12.16 and 12.17). The anatomy of the AV valves can be seen in this view, as well as in a sweep of the multiplane angle from 0 to 90° that incorporates both the ME RV In-Out and ME 2 Ch views. Alignment of the great vessels is dependent on their position; therefore no single TEE view will be adequate for all patients with DORV. The advantage of the multiplane probe is that the best view of the outflow tracts can be determined as the study is performed. The DTG LAX and DTG Sagittal views are also useful to visualize the great arteries and their relationship to the VSD (Fig. 12.19, Video 12.15). Additional VSDs can be viewed in the ME 4 Ch and ME RV In-Out views, with variation of multiplane angle between 0 and 90° and axial probe rotation to perform a complete sweep of the ventricular septum. The transgastric short axis views (TG Basal SAX, TG Mid SAX) are also useful to evaluate for additional VSDs. Other associated anomalies can be demonstrated using TEE, including AV canal defect (as is common in heterotaxy syndrome), as well as juxtaposition of the atrial appendages, which can be seen from a modified ME 4 Ch view as well as ME AV SAX view (Fig. 12.20, Video 12.16). Systemic venous connections can be evaluated with the LE Situs SAX and lower esophageal IVC long axis (LE IVC LAX) views, as well as the ME Bicaval view (Chap. 4). Pulmonary venous connections can be evaluated using the modified ME 4 Ch and ME Bicaval/ME 2Ch views, as described in Chaps. 4 and 6.
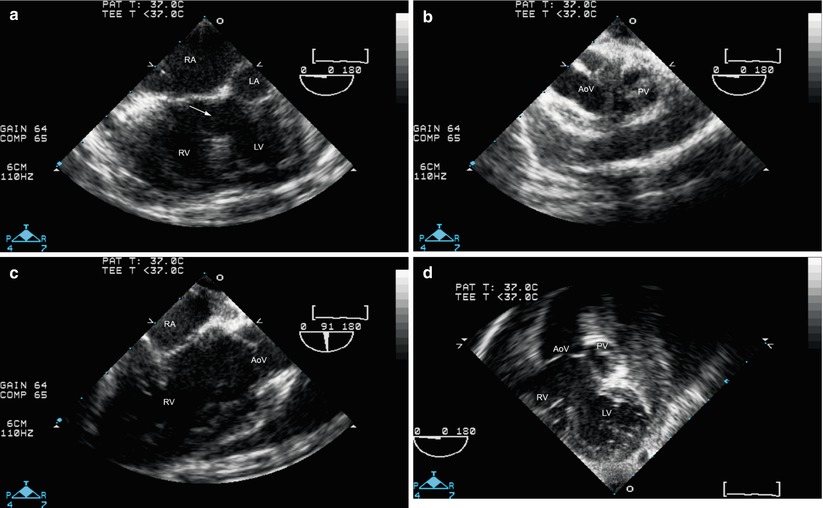
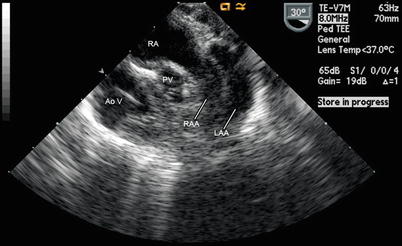

Fig. 12.19
Double outlet right ventricle with pulmonary outflow tract obstruction. Mid esophageal four chamber view (a) shows a large ventricular septal defect (arrow). With probe withdrawal and anteflexion, the semilunar valves are visualized (b). However the pathway from left ventricle (LV) to aortic valve (AoV) is not clearly shown. Rotation of multiplane angle to about 90° (c) shows right atrium (RA) and right ventricle (RV) as well as AoV, but it is still unclear whether the pathway from LV to AoV is unobstructed. The deep transgastric long axis view (d) shows that this pathway is unobstructed. This patient successfully underwent a patch closure of the ventricular septal defect and relief of pulmonary outflow tract stenosis.

Fig. 12.20
Left juxtaposition of the atrial appendages in a patient with double outlet right ventricle and pulmonary stenosis. This image was obtained from a mid esophageal aortic valve short axis view. Note how the right atrial appendage (RAA) crosses posterior to the great arteries to lie just anterior to the left atrial appendage (LAA). AoV aortic valve, RA right atrium, PV pulmonary valve
Color Doppler can be used to assess for preoperative AV and semilunar valve regurgitation as well as restriction of flow across the VSD. Color interrogation can also be useful to assess inflow into each ventricle, particularly when straddling or overriding AV valves are suspected. Pulsed and continuous wave Doppler assessment of outflow tract obstruction is dependent on the angle of interrogation. The DTG LAX and DTG Sagittal views can be very helpful for this purpose. LV systolic pressure estimate (as measured by mitral regurgitation jet) is important if a restrictive VSD is suspected. The LV may be at suprasystemic pressure in these instances. This method of assessment, performed in the ME 4 Ch and ME 2 Ch views, is particularly important when the VSD jet is not at an appropriate angle for interrogation.
Surgical Considerations
When both ventricles and both AV valves are well developed, the goal of surgical repair is to utilize the VSD to baffle the LV to one of the great arteries. If baffled to the aorta, a “physiologic repair” is achieved. If baffled to the pulmonary artery, an arterial switch operation is required. In cases where the VSD is remote from the great arteries, septation might not be possible. In addition, presence of a straddling tricuspid valve over a canal type VSD or straddling mitral valve over a malalignment VSD may also preclude septation into two ventricles. In these cases, staged surgical palliation culminating in the Fontan operation is usually performed [38].
For those undergoing a biventricular repair, selection of the appropriate operation depends upon an assessment of great artery relationship in relation to the ventricles and VSD, whether outflow tract obstruction is present and if so, which outflow tract is obstructed and to what degree. Based upon these assessments, a determination can be made as to the most suitable operation to effect a complete biventricular repair. The most common operations include:
Patch closure of VSD (baffle to the aorta).
Rastelli type procedure: Baffle closure of VSD to aorta, with repair of obstructed RV outflow tract (outflow patch or RV to pulmonary artery conduit).
Arterial switch operation with closure of VSD to neo-aorta.
Nikaidoh operation with translocation of the aorta into the pulmonary position over the LV, VSD closure and placement of an RV to pulmonary artery conduit [39, 40]. This operation is designed to bring the aorta and aortic valve closer to the LV, thus reducing the risk of postoperative LV outflow tract obstruction (Fig. 12.21, Video 12.17).
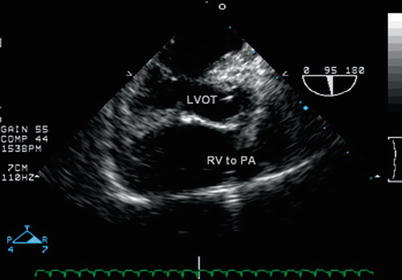
Fig. 12.21
Mid esophageal aortic valve long axis view in the patient from Figs. 12.16, 12.17, and 12.18 with double outlet right ventricle, posterior malalignment of the conal septum with subpulmonary ventricular septal defect after the Nikaidoh procedure (aortic translocation). The translocation of the aorta has placed the aortic valve closer to the left ventricle, improving left ventricle to aortic valve alignment and reducing the possibility of subaortic obstruction once the ventricular septal defect has been closed by a patch. This image demonstrates the “physiologic” repair achieved by baffling the ventricular septal defect to the aorta in its new position and the right ventricle to pulmonary artery conduit. LVOT left ventricular outflow tract, PA pulmonary artery, RV right ventricle
Postoperative assessment is dependent upon the surgical intervention. In those patients with a subaortic VSD, the two most important issues for intraoperative TEE are assessment for a residual VSD and determination of possible LV outflow tract obstruction from the baffle to the aorta. Subaortic conus as well as VSD patch position may result in significant subaortic obstruction. The best view to assess the LV outflow tract is variable, depending on the relationship of the aorta to the LV. Though the ME AV LAX view with a multiplane angle of 120° is best for a normal LV outflow tract, this may not be the case in DORV, thus trying different TEE views, probe rotations, and multiplane angles, will often be required to obtain the best image. Doppler interrogation of the outflow tracts can also be challenging. A variety of different TEE views will often be necessary to obtain the optimal Doppler angle of interrogation; these usually include a combination of mid esophageal, transgastric, and (when available) deep transgastric views. Severity of obstruction may also have to be determined by other methods such as smallest diameter. In some cases, direct pressure measurements in the LV and the aorta might be required.
Assessment for residual ventricular communications is very important in patients undergoing repair of DORV. Intramural type defects can occur in this lesion as well (See section “Tetralogy of Fallot”). In patients with Taussig-Bing anatomy that undergo the arterial switch operation, intraoperative TEE assesses adequacy of the neo-aortic and neo-pulmonic anastomoses (see section “Transposition of the Great Arteries”) as well as the adequacy of the repair of associated defects.
Truncus Arteriosus
Anatomy
Truncus arteriosus communis, also known more simply as truncus arteriosus (TA) or common arterial trunk, is characterized by the following anatomic features: a solitary great vessel arises from the heart, giving rise to the aorta, at least one pulmonary artery, and at least one coronary artery. Almost always, there is a large VSD in which the septal band (septomarginal trabeculation) forms the “floor” of the VSD, and the truncal root overrides the crest of the ventricular septum so that the solitary truncal valve forms the “roof” of the VSD [41]. The morphology of the VSD in TA is virtually identical in to that of TOF [42], however in TA the infundibular septum is absent. TA must be distinguished from TOF/PA. In TOF/PA a remnant of the main pulmonary artery and valve can often be seen. In the most extreme form of TOF/PA, the pulmonary circulation may be supplied directly from the aorta. Thus, the distinction between TOF/PA and TA is sometimes difficult. In TA, the solitary semilunar valve, the truncal valve, is often morphologically abnormal. Though a tricuspid truncal valve is the most common anatomic subtype (69 %), other variations can occur including (in order of frequency), quadricuspid (22 %), bicuspid (9 %), and rarely valves with five leaflets (0.3 %) [43]. Because of these frequent abnormalities in valvar anatomy, truncal valve stenosis and/or regurgitation are common, and if clinically significant, they may need to be addressed surgically. Approximately 25 % of patients with TA have a right aortic arch. Other associated cardiac anomalies include aberrant origin of a subclavian artery from the descending aorta, persistent left superior vena cava to coronary sinus connection, coronary anomalies, and an atrial communication in the form of a patent foramen ovale or secundum atrial septal defect. There have been rare reports of TA with single ventricle. In the absence of aortic arch obstruction a ductus is rarely seen in this lesion.
Two classification schemes have been proposed for this malformation: one described by Collett and Edwards [44], the other by Van Praagh [42, 45]. Both are based principally upon the manner in which the pulmonary arteries arise. The classifications share similarities although there are also some important differences (Fig. 12.22).
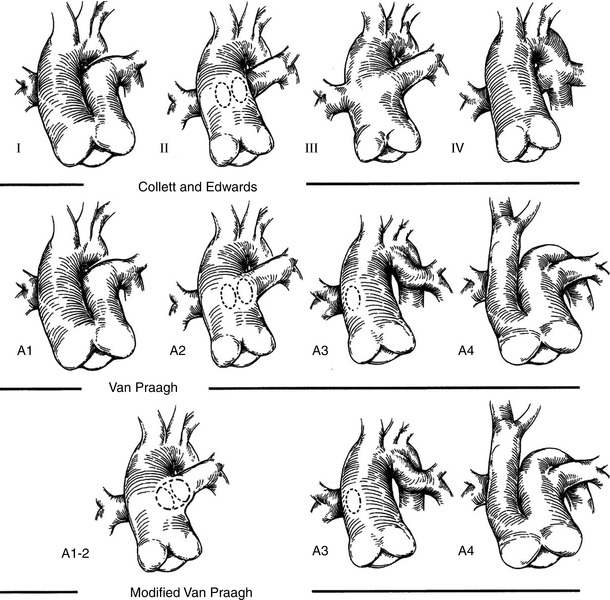

Fig. 12.22
The two common classification systems for truncus arteriosus: Collett and Edwards (first row) and Van Praagh (second row). A modified Van Praagh classification system is shown on the third row. See text for details (Reprinted from: Jacobs [46] with permission from Elsevier)
The Collett and Edwards classification is listed as follows:
Type 1: There is partial absence of the aorto-pulmonary artery septum, and a short main pulmonary artery segment (of variable length) that arises from the common trunk and gives rise to the branch pulmonary arteries. This is the most common type, comprising 48–68 % of all TA [43].
Type 2: There is total absence of the aorto-pulmonary septum and the pulmonary arteries arise directly from the posterior aspect of the common trunk. This type comprises 29–48 % of all TA [43].
Type 3: The branch pulmonary arteries arise separately from the common trunk and are remote from each other. This type comprises 6–10 % of all TA [43].
Type 4: The pulmonary circulation derives from the collateral arteries arising from the descending aorta. This type is now considered a form of TOF/PA with aortopulmonary collaterals, and is not generally used as a classification for TA.
The Van Praagh classification is listed below:
Type A1: There is partial absence of the aorto-pulmonary artery septum: there is a main pulmonary artery segment (of variable length) that arises from the common trunk and gives rise to the branch pulmonary arteries.
Type A2: There is total absence of the aorto-pulmonary septum and the pulmonary arteries arise directly from the posterior aspect of the common trunk.
Type A3: One of the pulmonary arteries arises directly from the common trunk and one arises directly from a collateral supply (usually the ductus arteriosus). This is also known as truncus with absent pulmonary artery.
Type A4: Truncus arteriosus in association with interruption of the aortic arch. The common trunk gives rise to the ascending aorta and a main pulmonary artery, from which the ductus arteriosus and the branch pulmonary arteries arise. The transverse arch is absent, thus there is an interrupted aortic arch. The ductus arteriosus provides blood to the descending aorta, thus this type of TA is a ductal-dependent lesion. The interruption is usually type B (between the left carotid and the left subclavian arteries). This type occurs in 13 % of cases.
Of note, the original Van Praagh classification provided a subclassification of each type: A (with a VSD) and B (no VSD). Thus a Type 1 TA could be divided into Type A1 and Type B1, depending upon the presence/absence of a VSD [42]. However given the fact that a VSD is almost always present, the Type B subclassifications are rarely seen. The reader will also note that the first two types of TA for both classification schemes are essentially identical; these are by far the common types of TA encountered. Another important point is that many TA patients are classified as “Type 1” when, in reality, the two pulmonary artery orifices are located so close together that no main pulmonary artery segment is present. This type is often referred to as “truncus arteriosus Type 1–2” or “Type 1½” (Fig. 12.22) [46].
TEE Evaluation
The pertinent aspects of cardiac anatomy and function that should be evaluated in a patient with TA includes the following:
Assessment of the VSD and evaluation for potential additional VSDs.
Evaluation of truncal valve anatomy and function.
Determination of the origin of both pulmonary arteries from the common trunk as well as their anatomy.
Evaluation of AV valve function.
Evaluation of ventricular systolic function.
Comparison of ascending aorta to main pulmonary artery size. In Type 1 TA (Type A1 of Van Praagh), the ascending aorta is generally much larger than the main pulmonary artery segment. However in TA patients with an interrupted aortic arch (Type A4 of Van Praagh), the ascending aortic diameter will usually be smaller than that of the main pulmonary artery [42, 45, 49].
Assessment of other possible cardiac abnormalities, including atrial septal defects, systemic/pulmonary venous anomalies, and possible coronary artery anomalies.
TEE can be very helpful in the pre-operative evaluation of the patient with TA. A careful TEE study can be used to evaluate all of the elements listed above. The ME 4 Ch view provides for evaluation of AV valve function and global assessment of ventricular performance. Withdrawal of the probe with anteflexion will demonstrate the truncal valve. Forward rotation of the multiplane angle to 30–45° will often reveal the truncal valve anatomy en face (Fig. 12.23, Video 12.18). Similar to all conotruncal defects, complementary TEE views (modified ME 4 Ch, ME Bicaval, LE Situs SAX and LE IVC LAX) establish the systemic venous connections and confirm the presence of an atrial communication, if present. The VSD can be visualized in orthogonal planes in the ME 4 Ch view (using probe anteflexion) or the ME AV LAX view (Fig. 12.24, Video 12.19). In Type 1 or 2 TA, the mid ME Asc Ao SAX and mid esophageal ascending aortic long axis (ME Asc Ao LAX) views, will often demonstrate the pulmonary arteries originating from the posterior aspect of the trunk (Fig. 12.24, Video 12.19). It can be difficult to see the entire length of the branch pulmonary arteries by TEE, but significant portions can be visualized using the aforementioned TEE views as well as the upper esophageal views (UE PA LAX, UE Ao Arch SAX).
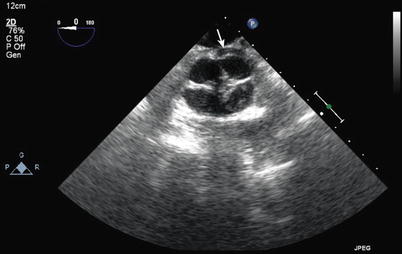
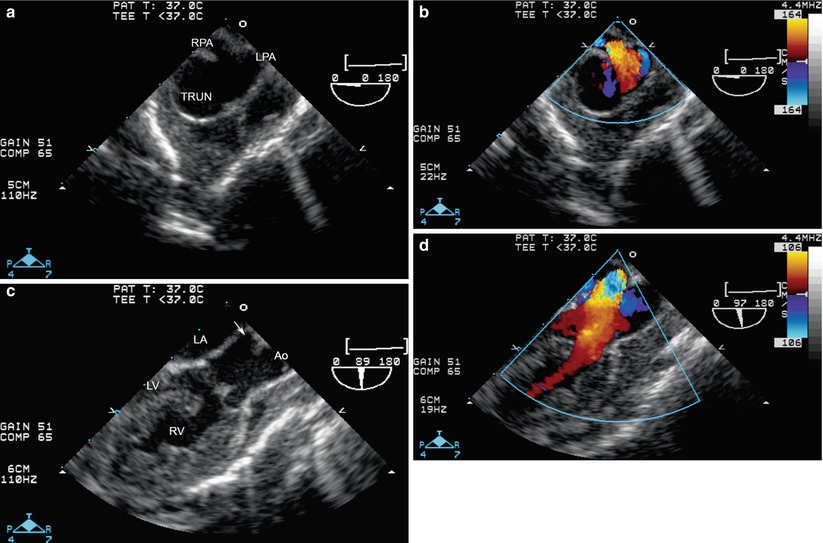

Fig. 12.23
Truncal valve seen en face from a modified mid esophageal aortic valve short axis view. This shows a quadricuspid truncal valve with thickened edges and a central area of noncoaptation. Note the left main coronary artery arising from the rightward, posterior cusp (arrow)

Fig. 12.24
Truncus arteriosus Type “1½” as seen from the mid esophageal ascending aortic short axis (a, b) and mid esophageal aortic valve long axis (c, d) views. From the short axis view, the right pulmonary artery (RPA) and left pulmonary artery (LPA) origins are seen immediately adjacent to each other, arising from the posterior aspect of the trunk (TRUN). From the long axis view this posterior origin of the pulmonary arteries is also seen (arrow), and the truncal valve shown to override the ventricular septal defect. Ao ascending aorta, LA left atrium, LV left ventricle, RV right ventricle
It is important to consider that TEE probe placement can be difficult in small infants with TA especially when there is associated 22q11 deletion syndrome (Chap. 3).
Surgical Considerations
Surgery for TA is usually performed in the neonatal period because most patients become symptomatic with congestive symptoms and pulmonary vascular obstructive disease can develop early. The type of surgical approach is dictated by the truncal anatomy. For the common forms of TA (Types 1 and 2) surgical repair consists of closure of the VSD to the truncal valve, establishing continuity between the LV and truncal valve, removal of the pulmonary arteries from the common trunk, and in most cases, placement of a conduit between the RV and the pulmonary arteries. In TA Type A4 (of Van Praagh), the interrupted arch is addressed in addition to VSD closure and RV to pulmonary artery conduit, as noted above. For all TA repairs, if the truncal valve manifests significant stenosis and/or regurgitation, surgical intervention might be necessary.
Postoperative TEE helps determine adequacy of the repair. The goals of this imaging are to assure that there is unobstructed flow from the LV to the truncal root, no significant residual VSD and unobstructed flow from the RV to the branch pulmonary arteries. Evaluation of the interatrial septum should be undertaken for residual shunting. It should be noted that in some cases, an intentional small interatrial communication remains, allowing for potential right to left shunting and the maintenance of cardiac output during the immediate postoperative period. In addition, the postbypass examination is of benefit in the evaluation of truncal valve function (estimation of residual severity of obstruction and/or regurgitation) in cases where concomitant valve interventions were performed.
The ME 4 Ch view sweeping from inferior to superior demonstrates that the VSD patch is in the correct position and color flow Doppler can be used to determine whether there is residual shunting (Fig. 12.25, Video 12.20). The intramural type VSD previously described can also occur with TA repair [23]. Assessment for a residual VSD should always occur in more than one view. The ME RV In-Out view is quite useful to demonstrate a peri-patch residual leak. In addition, the RV to pulmonary artery pathway is best seen in this view with a counterclockwise turn of the probe toward the left side of the patient. Doppler interrogation of this pathway can also be performed to assess for outflow tract obstruction. The LV to truncal valve pathway can be seen well in the ME AV LAX view along with assessment of truncal stenosis and regurgitation (Fig. 12.26, Video 12.21) [50]. When available, the deep transgastric views (DTG LAX, DTG Sagittal) can provide visualization of the LV outflow tract and proximal RV to pulmonary artery conduit, as well as excellent angles of insonation for spectral Doppler assessment. The ME 4 Ch and ME Bicaval views can be used to assess the atrial septum, and the ME 4 Ch, ME RV In-Out, and ME 2 Ch views can facilitate the evaluation AV valve function.
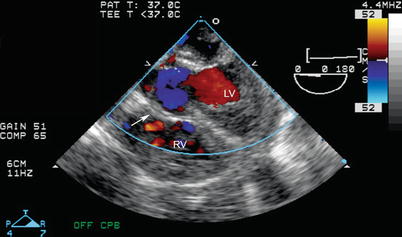
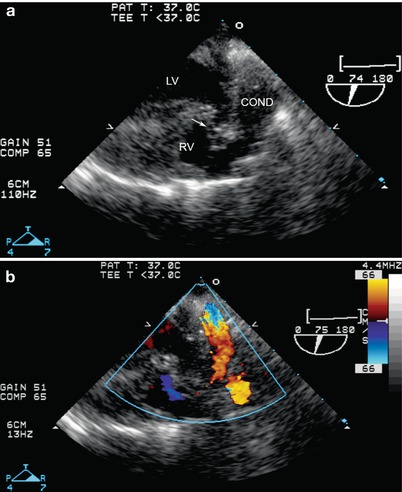

Fig. 12.25
Postop truncus arteriosus repair, seen from mid esophageal four chamber view with probe anteflexion. The ventricular septal defect patch is shown by the arrow; no residual defect is present by color flow imaging. LV left ventricle, RV right ventricle,

Fig. 12.26




Postop truncus arteriosus repair, seen from a modified mid esophageal aortic valve long axis view, multiplane angle about 75°, with imaging (a) and color flow Doppler (b). The ventricular septal defect patch (arrow) is seen as well as the origin of the conduit (COND) from the right ventricle (RV) to the pulmonary artery. LV left ventricle
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


