Reference
HR
Men (n)
CVD (%)
Hypertension (%)
Mean follow-up (years)
All-cause mortality/CVD
Shores et al. [23]
1.88
858
20.7
NA
8.0
All cause
Khaw et al. [24]
2.29
2,314
0
20
10
All cause and CVD
Laughlin et al. [25]
1.40
794
35
75
20
All cause
1.38
CVD
Tivesten et al. [26]
1.65
3,014
26.5
NA
4.5
All cause
Vikan et al. [27]
1.24
1,568
17
NA
11.2
All cause
Haring et al. [28]
2.32
1,954
9
29
7.2
All cause
2.56
CVD
Malkin et al. [29]
2.27
930
100
42.6
6.9
All cause
Menke et al. [30]
1.43
1,114
0
NA
9.0
All cause
Corona et al. [31]
7.10
1,687
11.6
27.4
4.3
CVD
Vlachopoulos et al. [32]
3.83
228
0
100
3.8
CVD
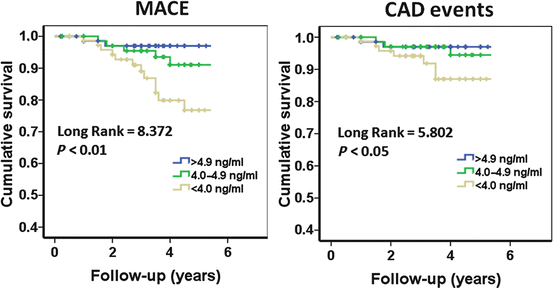
Fig. 4.1
Kaplan-Meier curves for major adverse cardiovascular events (MACE) and coronary artery disease (CAD) events by tertile group of total testosterone (TT). Cut-offs of the TT tertiles were 4.0 and 4.9 ng/mL (With permission from Vlachopoulos et al. [32])
4.3.2 Testosterone and Target Organ Damage
Screening for subclinical organ damage is of paramount importance, because asymptomatic alterations of the cardiovascular system and the kidney are crucial intermediate stages in the disease continuum that links hypertension to cardiovascular events and death [39]. Low testosterone may be implicated in the common pathogenetic pathways of ED and CVD through changes in vascular function and structure. Testosterone has been reported to inhibit vascular smooth muscle cell proliferation and neointima formation, suggesting a direct action of testosterone on the vasculature [40]. Indeed, testosterone has been associated with increased arterial stiffness [41], carotid intima-media thickness (IMT) [42], and ankle–brachial index [43]. Interestingly, in a recent study we reported a more prominent effect of testosterone deficiency on aortic stiffness in young men and in subjects with higher blood pressure levels (Fig. 4.2) [41]. This finding identifies testosterone as a marker of arterial damage with special emphasis on young and hypertensive individuals and supports its role as predictor of events. Of particular importance is the finding that aortic stiffness is a potent biomarker within the context of ED that identifies patients who are at higher risk [44]. Finally, changes in cardiac function may also be involved: low testosterone has been associated with left ventricular hypertrophy [6].
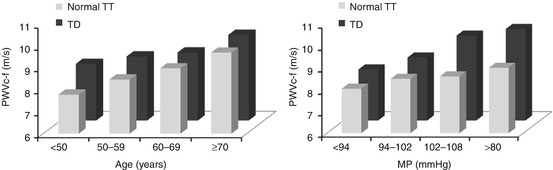

Fig. 4.2
Impact of the relation between (a) age groups, (b) BP (mean pressure) groups, and testosterone deficiency (TD) on carotid-femoral pulse wave velocity (PWVc–f). In younger categories (<50 year and 50–59 years), patients with TD had higher blood pressure-adjusted PWVc–f compared to subjects with normal TT, indicating an “aging effect” of 10 years, whereas in older age categories such a difference was not observed. In men with a higher mean pressure (102–108 mmHg and >108 mmHg), patients with TD had higher age-adjusted PWVc–f compared to subjects with normal TT, indicating a synergistic unfavorable effect of TD and blood pressure on aortic stiffness (With permission from Vlachopoulos et al. [41])
4.4 Measurement of Testosterone in the Evaluation of ED Hypertensive Men
In the ED patient, measurement of testosterone levels is justified to be included in the clinical evaluation of the patient because it serves the following purposes: (1) to assist in the diagnosis of ED, discriminate the cause of ED, and estimate the severity of ED; (2) to identify patients at higher cardiovascular risk and predict CV events in ED patients (i.e., to predict CVD incidence); (3) to identify hypertensive males with subclinical organ damage; and (4) to enhance the ability of the clinician to optimally choose the correct treatment for patients in whom phosphodiesterase-5 (PDE5) inhibitors have failed [45–47]. Importantly, it has been integrated as first tool in the most recent, third (2012) Princeton Consensus for the assessment and management of patients with hypertension of organ ED [48].
4.5 Testosterone Therapy
To whom: Testosterone therapy (TTh) should be reserved for patients who (i) are symptomatic (ED or reduced libido) of testosterone deficiency and (ii) they have biochemical evidence of low testosterone (TT <8 nmol/L or 2.3 ng/mL) [49]. In men with borderline TT levels (8–12 nmol/L or 2.3–3.5 ng/mL), a TTh trial (for 3–6 months and continuation if effective) may be envisaged. Improvement is dependent on the testosterone levels with best results being obtained when TT is below 10.4 nmol/L (3 ng/mL). Men treated with testosterone require long-term, careful follow-up. There should be careful follow-up of men with severe obstructive sleep apnea and men with severe hypertension, due to the risk of fluid retention in case of overdosing [49].
Effect on BP: Currently, available evidence derived from a meta-analysis of 30 trials supported a neutral effect of testosterone supplementation on systolic (0.8 mmHg; 95 % CI, −4 to 5 mmHg, P = NS) and diastolic (2 mmHg; 95 % CI, −2 to 6 mmHg, P = NS) BP that was consistent across trials [50]. However, in a recent prospective, observational study of the use of injectable long-acting testosterone undecanoate in men during routine clinical care (of them, 26 % were hypertensives), blood pressure changed in a significant and favorable manner [51]. Data on the impact of TTh on BP levels in treated and untreated hypogonadic essential hypertensive patients with no other conditions or risk factors are not yet available.
Effect on lipids: The majority of prospective clinical studies indicate that TTh within physiologic limits has beneficial or neutral effects on lipid profile other than HDL-C, beneficial or neutral effects on inflammatory mediators, and generally beneficial effects on glycemic state [52, 53]. Lean body mass is typically increased in hypogonadal subjects, and visceral adiposity is decreased in several studies and unchanged in the remainder [54]. Such metabolic effects have raised interest on the potential impact on cardiovascular health.
Effect on TOD: Regarding the effect of TTh on markers of organ damage, a decrease in aortic stiffness measured as pulse wave velocity was evident after 48 weeks; likewise, a decrease in large artery compliance was found after 3 months [55]. A reduction on carotid artery intimal thickness was observed at 12 months in one study, but this did not reach significance because of the small cohort size [56]. The effect of TTh in men with left ventricular hypertrophy or microalbuminuria has not been examined yet.
Effect on CV risk: Most studies have shown neutral [57] or even beneficial [58] effect of TTh on cardiovascular risk. However, in two recent studies there was an increase in cardiovascular events with TTh [59, 60]. In both of these studies, hypertension was highly prevalent (~90 %). The increase in adverse events with TTh reported in the Testosterone in Older Men with Mobility Limitations (TOM) trial may have been related to a high rate of comorbidities and the use of high and rapid escalation dosing regimens [59]. The study by Vigen et al. retrospectively compared rates of death, myocardial infarction, and stroke from a dataset of 8,709 men who had undergone coronary angiography with prior documentation of serum T concentration <300 ng/dL; however, credibility of this article is deeply compromised by major errors, and subsequent attempts to correct, in values presented [60]. It cannot be overemphasized, therefore, that prospective data from large, well-designed, long-term trials of TTh in hypertensive males without clinical atherosclerosis are warranted.
4.6 Conclusions
Human studies indicate that men with lower testosterone levels tend to have higher blood pressure levels and incidence of hypertension. Testosterone deficiency is a common pathogenetic mechanism linking hypertension and vasculogenic ED. The relationship between hypertension and ED may also be due to reductions in testosterone levels due to antihypertensive drugs such as β-blockers and spironolactone that are known to affect the sexual function in hypertensive men on therapy. Emerging evidence supports that lower androgen levels are associated with indices of organ damage and predict poor cardiovascular risk profile. Measurement of testosterone also allows better prediction of CV risk compared with conventional risk assessment and identifies high-risk individuals in whom a more intense treatment is needed. Increased recognition of the potential of looking for testosterone deficiency in hypertensive men with ED, followed by appropriate replacement therapy, can improve sexual function and may decrease blood pressure levels and affect incidence of adverse CV events. We recommend that measurement of testosterone is part of cardiovascular risk assessment in all hypertensive males.
References
1.
2.
Kaushik M, Sontineni SP, Hunter C (2010) Cardiovascular disease and androgens: a review. Int J Cardiol 142:8–14PubMedCrossRef
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


