Metabolic disease
Diabetes mellitus
Chronic kidney disease
Hypogonadism
Hyperprolactinemia
Hypertension
Obesity
Metabolic Syndrome
Dyslipidemia
Liver, Lung and cardiovascular disease
Neurologic disease
Spinal Cord injury
Brain injury
Parkinson disease
Multiple sclerosis
Alzheimer disease
Stroke
Vascular disease
Aging, smoking, and lack of physical exercise
Psychogenic causes
Drug related
Most of the risk factors for ED are also at work for patients with chronic kidney disease. The ESRD population is currently increasingly elderly; moreover diabetes and hypertension are major causes of ESRD requiring dialysis. Older age, hypertension and diabetse mellitus have been found as independent correlates of ED in patients with ESRD [4]. Factors related to ED in uremic patients include risk factors of ED observed in the general population, plus some additional risk factors specific to the CKD population. The latter include anemia [9], and volume overload [10]. Depression has also been determined as a strong independent predictor of ED in dialysis patients.
Hypogonadism as a Cardiovascular Risk Factor
Epidemiology
Association with Cardiovascular Disease
Male gender has been established as a cardiac risk factor and the culprit has been traditionally attributed to testosterone hormone. However, recent evidence suggest the opposite: deficiency of testosterone may be responsible for increased cardiovascular risk [13]. Epidemiologic data relates testosterone deficiency with increased mortality risk. In a case–control study Khaw and colleagues [14] found that testosterone was inversely associated with all-cause and cardiovascular mortality. Pye et al. [15] evaluated prospective data from the European Male Aging Study (EMAS) on 2,599 community-dwelling men aged 40–79 years in eight European countries. Fifty-five men (2.1 %) were identified as having late-onset hypogonadism. After adjusting for age, body mass index, smoking, and poor general health, men with severe hypogonadism had a 5-fold higher risk of all-cause mortality compared with men without hypogonadism. In a meta-analysis of community-based studies, Araujo et al. [16] found that low endogenous testosterone levels are associated with increased risk of all-cause and CVD death.
Similar trends have been reported among patients with CKD. Yilmaz et al. [17] found that the risk of composite CV events was reduced by 22 % for each 1 nmole/L increment of serum total testosterone level in predialysis CKD patients. In another study conducted on predialysis CKD patients, the presence of stage 3–5 CKD and low testosterone levels were found to be additive risk factors for mortality [18]. Similar findings were also reported for patients undergoing hemodialysis [19].
Pathophysiologic Mechanisms
Hypogonadism is associated with numerous but mild symptoms, hence it is commonly underdiagnosed. Main manifestations of the disorder include, but are not limited to, reduced energy, mood changes, decreased libido, erectile dysfunction, fatigue, increased visceral fat, loss of muscle tissue. In addition to these protean manifestations, recent studies showed a strong association between hypogonadism and atherosclerosis. The difficulty in evaluation of the relationship stems from sharing of most of the risk factors by the two disorders.
In a population-based cross-sectional study, Svartberg et al. [20] found that lower levels of testosterone in men were associated with higher blood pressure levels and left ventricular hypertrophy. Moreover, replacement of testosterone in hypogonadal men led to decreases in blood pressure. Several mechanisms have been put forward [21].
Epidemiological data suggest that serum testosterone levels are inversely associated with total cholesterol, LDL cholesterol and triglycerides. However, HDL cholesterol also shows a positive correlation with testosterone levels [22]. Some studies reported that testosterone replacement resulted in a decrease in total cholesterol and LDL cholesterol levels, whereas the impact on HDL cholesterol was not that clear [23–25].
Low testosterone levels have been associated with metabolic syndrome and type-2 diabetes mellitus. Stellato and colleagues [26] evaluated the effect of serum testosterone on development of de novo diabetes mellitus. The authors reported that after controlling for potential confounders, diabetes at follow-up was independently predicted by lower baseline levels of free testosterone and sex hormone binding globulin (SHBG). Another study also demonstrated that testosterone and SHBG could predict the development of metabolic syndrome and diabetes in middle-age men [27]. Concentrations of SHBG and total and calculated free testosterone were determined at baseline in 702 middle-aged Finnish men participating in this population-based cohort study. After 11 years of follow-up, men with total testosterone, calculated free testosterone, and SHBG levels in the lower fourth at baseline had a 2.3 odds ratio of developing each for the metabolic syndrome and diabetes mellitus.
SHBG is considered as the probable mediator of this increased risk. Rajala and colleagues [28] found that increased risk of insulin resistance was independently associated with SHBG but not with total testosterone level. Thus the relationship between diabetes mellitus and metabolic syndrome development was stronger for total testosterone than for free testosterone. Moreover, iatrogenic androgen deprivation in patients with prostate cancer is associated with hyperglycemia and metabolic syndrome.
Additional confirmation of this association came from interventional studies. Six-month treatment of hypogonadal men with type II diabetes mellitus and metabolic syndrome with testosterone replacement resulted in improved glycemic control and body composition, compared with the control group [29]. Testosterone replacement therapy reduced HOMA-IR in the overall population by 15.2 % at 6 months and 16.4 % at 12 months. The mechanism of this beneficial effect of testosterone seems to be due to improved insulin resistance rather than direct effects of testosterone on the pancreas.
There is a bidirectional relationship between obesity and hypogonadism. In a population cohort study, Laaksonen et al. [30] showed that men with metabolic syndrome had a 2.6 fold increased risk of developing hypogonadism after 11 years of follow-up, independently of age and other potential confounders. In contrast androgen deprivation studies showed that within a few months after treatment onset, body fat accumulates [31] and there is an increased incidence of new diabetes mellitus type II cases [32].
The relationship between obesity/diabetes and hypogonadism is likely mediated by inflammatory cytokines secreted by the visceral fat tissue. Androgen deprivation therapy is associated with increased levels of proinflammatory and decreased levels of anti-inflammatory cytokines [33, 34]. Schroeder et al. [35] conducted a placebo-controlled study in which they administered oxandrolone to the treatment group for 12 weeks. Compared with placebo treated men, androgen treated cases showed significant and durable reductions in regional abdominal and peripheral adipose tissues along with improved insulin sensitivity. Although the latter study did not measure specific proinflammatory cytokine levels, it may be speculated that level of these markers might have been reduced with the androgen treatment based on the observed reduction in abdominal fat tissue and improved insulin sensitivity since both of which are surrogate markers of increased inflammation.
Endothelial tissue has receptors for androgens. Administration of testosterone results in dilatation of pulmonary and coronary arteries [36]. Oral testosterone treatment resulted in increased myocardial perfusion in hypogonadal men with CAD [37]. Low testosterone level was found to be associated with endothelial dysfunction [38]. In a randomized controlled trial, long-term oral testosterone supplementation in patients with CAD improved brachial artery vasoreactivity [39]. It’s believed that testosterone leads both endothelial dependent and endothelial independent vasodilatation (Fig. 16.1) [40, 41].
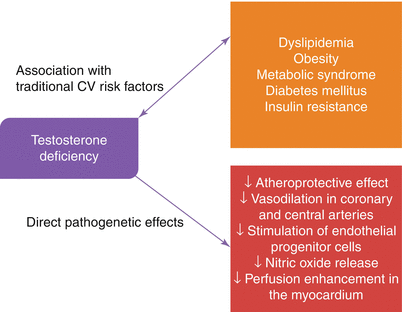

Fig. 16.1
Pathophysiologic effects of testosterone deficiency
Hypogonadism in Chronic Kidney Disease: Causes and Consequences
The kidneys are endocrine organ as both a main modulator of endocrine function and major target for hormonal action [42]. Thus chronic kidney disease may affect several hormonal axes. These hormonal changes are depicted in Fig. 16.2. Several factors in addition to hormonal derangements related to kidney dysfunction itself contribute to the hypergonadotropic hypogonadism seen in CKD. Potential contributor factors are shown in Fig. 16.3.
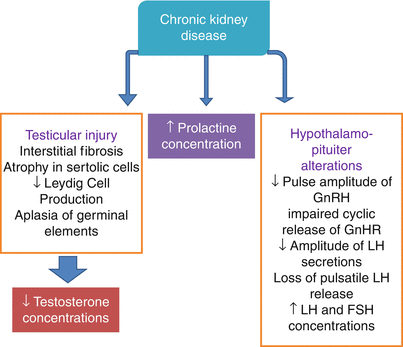
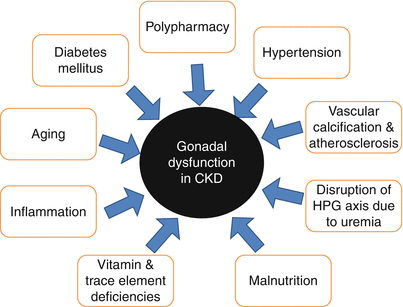

Fig. 16.2
Abnormalities in hormonal functions of HPG axis in patients with CKD

Fig. 16.3
Potential contributory factors of hypergonadotropic hypogonadism of CKD
Testosterone appears to be the pivotal mediator of increased cardiovascular risk in patients with hypogonadism. Plasma total and free testosterone as well as 5α-dihydrotestosterone levels are decreased in ESRD patients. Normalization of serum testosterone and LH levels after successful renal transplantation suggests the central role of uremia in hypergonadotropic hypogonadism [43]. In contrast to renal transplantation, other renal replacement therapies – such as hemodialysis and peritoneal dialysis, do not restore HPG axis. In addition, abnormal gonadotropin responses to GnRH are not corrected by hemodialysis whereas kidney transplantation improves the response [44].
Hypogonadism in CKD has been implicated in a number of complications. These include anemia, sexual dysfunction and erectile dysfunction, progression of CKD and cardiovascular morbidity and mortality [42].
Erectile Dysfunction-Cardiovascular Disease Nexus
Hypogonadism is just a one contributory factor among many others of erectile dysfunction. Low testosterone levels interacts closely with major atherosclerotic risk factors as described above. On the other hand, ED comprises a wide range of causative factors and associates in addition to hypogonadism. Unsurprisingly, similar to hypogonadism, ED as a whole is independently and strongly related to cardiovascular events. It is likely that endothelial dysfunction and increased inflammation and in many cases hypogonadism are at work leading to erectile dysfunction as well as concomitant coronary atherosclerosis. Compared with difficult to discern and nonspecific symptoms of hypogonadism, ED is apparent and thus, may be used as a early warning sign for simultaneous undiagnosed or future development of cardiovascular disease.
Association of ED with CAD
Several studies investigated frequency of ED in patients with CV disease primarily based on the concomitant risk factors of both disorders. These studies showed an increased prevalence of ED in patients with CV disease. In a seminal study, Montorsi and colleagues [45] reported that 50 % of patients who presented with chest pain and angiographically documented CAD had ED.
Vlachopoulos et al. [46] in a recent meta-analysis evaluated 14 longitudinal studies which looked at the relationship between presence of ED and CV events including mortality (92 757 participants; mean follow-up, 6.1 years). The authors concluded that ED is associated with increased risk of CV events and all-cause mortality. Relative risk was higher at younger ages, and in intermediate-risk groups.
The association between erectile dysfunction and cardiovascular disease has long been recognized. This association was first attributed to sharing of common risk factors for both disorders. Many major CV disease risk factors are at the same time risk factors for ED. These include age, sedentary life-style, smoking, metabolic syndrome, obesity, hypertension, dyslipidemia and diabetes mellitus [47]. However, large epidemiologic and prospective studies established the presence of erectile dysfunction as an independent risk factor for cardiovascular disease. Endothelial dysfunction, inflammation and hypogonadism (or low testosterone levels) have been suggested as the common denominators of both disorders [48–50]. Actually both ED and CV disease share abnormalities in nitric oxide pathway resulting in endothelial dysfunction in the early phase and structural atherosclerotic plaque development in the long-standing disease. It has also been suggested that ED may be the clinical equivalent of angina pectoris, as different manifestations of the same generalized vascular pathology [51].
Normal erectile function is a neurovascular event but modulated also by psychological and hormonal factors. On the other hand, vascular integrity plays a pivotal role in healthy erectile function. The initial abnormal endothelial function transforms into structural changes in small arteries supplying penis just as the case in coronary arteries supplying the myocardium. These structural changes in turn limits the blood supply after a critical occlusion level reached [52]. Montorsi et al. proposed the artery-size hypothesis as a possible explanation of why in some patients ED precedes development of cardiovascular events by 2–5 years [53].
If ED Is Severe, CAD Is Also Severe
The severity of ED has been related to the extent of coronary artery disease by several modalities. A prospective population-based Australian study (the 45 and Up Study) evaluating data of 95,038 men aged ≥45 years found that risk of CVD and death increased steadily with severity of ED. Among men without previous CVD, those with severe versus no erectile dysfunction had significantly increased risks of ischaemic heart disease (adjusted relative risk [RR] = 1.60), heart failure (8.00), peripheral vascular disease (1.92), all CVD combined (1.35), and all-cause mortality (1.93). Moreover, these risks were independent of major traditional cardiovascular risk factors. ED severity assessed by IIEF score was inversely correlated with angiographically proven CAD burden [54]. Multivessel CAD and higher calcification scores were more common among patients with more severe ED [55, 56].
ED and Prediction of CV Events
Erectile dysfunction usually precedes the onset of CAD. Several studies reported this time interval as 2–3 years in CAD symptoms and 3–5 years in cardiovascular events [45]. This relatively early presentation of ED compared with CAD symptoms and CV events has been accounted for by some hypotheses; Artery-size hypothesis suggest early occlusion of smaller penile arteries compared with larger diameter coronary counterparts by the same size atherosclerotic plaque. Another hypothesis suggests that in the younger person with ED impaired vasodilatation of penile arteries is more likely to result in ED than he develops angina due to impaired vasodilatation in coronary arteries [57].
Interestingly, ED is a better predictor of future CV events in younger males aged 40–70 years. In contrast, the prognostic importance of ED in order males >70 years of age is much less powerful.
Inclusion of ED as a risk factor of CAD has been studied in a number of risk prediction models. Incident ED has a predictive value for CV events that is similar or greater than that of smoking dyslipidemia and family history of coronary artery disease [58, 59]. Only one study evaluated addition of ED to Framingham Risk Score (FRS) to date. Araujo et al. [60] found that adding ED to FRS in a population based study including 1,057 males without CVD and diabetes and 40–70 years old did not improve prediction of CV events during 10 year follows up.
Cardiovascular Disease in CKD
Kidney disease is associated with increased CV disease risk. Both proteinuria and reduced GFR are associated with increased risk. A meta-analysis of population based studies reported that compared with eGFR 95 mL/min/1.73 m2, adjusted HRs for all-cause mortality were 1.18 for eGFR 60 mL/min/1.73 m2, 1.57 for 45 mL/min/1.73 m2, and 3.14 for 15 mL/min/1.73 m2. Similarly albumin to creatinine ratio was also associated with increased risk [61]. This increased risk was also true for specific patient groups such as patients with previous CV disease, hypertension, diabetes or combinations of these [62, 63].
National Kidney Foundation and the American College of Cardiology/American Heart Association to recommend that CKD be considered a CHD risk equivalent [64, 65]. Traditional cardiovascular risk factors such as older age, hypertension, diabetes, smoking, and dyslipidemia are prevalent in patients with CKD. In addition to these factors some nontraditional or novel risk factors also affect the risk of CV disease in CKD population. These nontraditional risk factors include, but not limited to, uremia, anemia, increased inflammation, endothelial dysfunction, vascular calcification, abnormalities in bone mineral metabolism, and malnutrition-inflammation complex [66, 67]. Several cross-sectional studies have suggested that the Framingham risk scoring is insufficient to capture the extent of CV disease risk in subjects with CKD [68]. This can be explained as effects of nontraditional risk factors and different interaction of traditional risk factors with CV disease risk in CKD patients than general population [64].
Approach to ED as an Harbinger of CV Disease
Since ED has been established as an independent marker of CV disease risk, and can be easily documented with patient interview, ED should be questioned in every patient who is evaluated for overall cardiovascular risk [47]. This is particularly more important in younger patients with seemingly vasculogenic ED.
The Princeton III Consensus offered recommendations for the evaluation and management of cardiovascular risk in men with ED and no known cardiovascular disease, with special emphasis on identification of men with ED who may require additional cardiologic work-up [69]. This report recommends that the initial risk stratification should be based on Framingham Risk Score in patient without prior known CV disease. This initial step stratify patients into three risk groups, namely low risk (≤5 %), intermediate risk (5–20 %) and high risk (≥20 %). High risk patients and symptomatic patients should be referred to cardiologists. Intermediate risk patients are advised to undergo exercise stress test. Patients with an abnormal exercise stress test should be referred to cardiologists. If the test result is negative, carotid intima media thickness, ankle brachial index or coronary calcium scoring can be considered as the next step. All abnormal results should be evaluated by a cardiologist. Patient with low risk and normal noninvasive cardiac workup need risk factor management [47]. Risk factor management and interventions which may prevent CV events are summarized in Table 16.2.
Table 16.2
Risk factor management and interventions which may prevent CV events in patients with ED
Modification of lifestyle factors |
Weight loss |
Increased physical exercise |
Decreased caloric intake |
Management of hypertension, diabetes mellitus, and dyslipidemia |
Medication issues |
Some medications associated with development or exacerbation of ED |
Beta-blockers |
Thiazides |
Calcium channel blockers |
Statins and fibrates |
ACE inhibitors |
Statins and angiotensin receptor blockers may be associated with improvements in ED |
ED at Crossroads Between CKD and CV Disease
Cardiovascular disease is the primary cause of death in patients with CKD. This is so starting from the early stages of the disease. On the other hand ED is also considerably common particularly in patients undergoing dialysis treatment. Since CKD patients suffer from an unproportionately high CV disease burden, early detection and markers which enable us to detect cardiac disease would be invaluable to prevent premature death from CV disease. ED offers such a opportunity in the general population behaving a sort of early warning sign of future CV events. However, it is still not known ED also provides such a predictive data in patients with CKD. This is a paramount clinical need in this vulnerable patient population.
Endothelial dysfunction and increased inflammation are inevitable components of advanced kidney disease. And both of them play fundamental roles in the development of atherosclerosis and erectile dysfunction (Fig. 16.4). What is not known right now is which comes first. Because kidney disease leads to somehow different pathologic consequences in the vascular structures. Dialysis patients with ischemic heart disease may not necessarily have large-vessel occlusive coronary disease. One study [70] showed that up to 50 % of nondiabetic dialysis patients with symptoms of myocardial ischemia did not have large-vessel coronary artery disease. Thus, The artery size hypothesis which accounts for the time lag between presentation of ED and angina in the general population may not be applicable here in CKD patients. Moreover, these patients also have left ventricular hypertrophy and cardiomyopathy which may make the issues more complicated. In addition, Framingham Risk Scoring has been shown to be not that effective in risk prediction in patients with advanced kidney disease [71]. Thus, efforts which try to implement ED as a major CV disease risk marker into commonly used risk prediction scores may not be adequately feasible in patients with kidney disease.
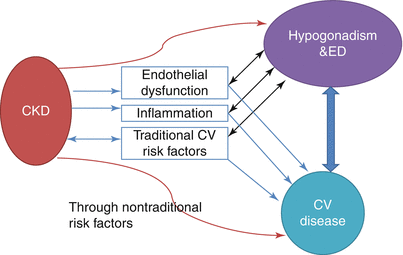

Fig. 16.4
Major pathophysiologic denominators and relationship among CKD, CV Disease and hypogonadism/ED
Patients undergoing hemodialysis also have high rates of depression [72, 73]. Depression is a cardinal cause of psychogenic ED. When we attribute ED of a patient to psychogenic ED primarily, we may have missed an opportunity to investigate underlying silent cardiovascular disease. Although depression is so prevalent, these patients are also subject to traditional and nontraditional CV risk factors which are common in dialysis patients. Thus, what is recommended in the general population in which one first should differentiate psychogenic from organic ED may again be ineffective in dialysis population.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


