Fig. 4.1
Annual number of admissions per 100,000 persons for chronic mesenteric ischemia in the Nationwide Inpatient Sample
Additionally, review studies estimate that approximately 10 % of CMI cases are attributable to etiologies other than atherosclerosis including primary vascular conditions such as dissection, fibromuscular dysplasia, or vasculitis; or other systemic conditions such as neurofibromatosis [12–16]. Dissection secondary to segmental arterial mediolysis (SAM), a non-atherosclerotic, non-inflammatory arteriopathy, with subsequent development of CMI, should also be recognized among these non-atherosclerotic causes though it is among the most rare with only 47 cases reported through 2011 [17]. The demographics of patients with non-atherosclerotic CMI vary widely according to the specific underlying pathology with most data restricted to single-center case series.
Natural History
The natural history of CMI has largely been elucidated by two prospective studies on the clinical course of patients found to have moderate to severe mesenteric atherosclerotic disease. From 1989 to 1995, Thomas and colleagues evaluated 980 aortograms of patients without CMI symptoms to identify 82 patients (8.3 %) with greater than 50 % stenosis of one or more mesenteric vessels [18]. These patients were then followed at six-month intervals with questionnaires on symptom status for a mean of 2.6 years (range: 1–6 years). Sixty patients meeting study criteria were available for follow-up during which time 4 patients (6 %) developed symptoms of mesenteric ischemia, 3 chronic and 1 acute. Notably, each of these 4 patients was among the 15 patients with three-vessel disease, while none of the remaining 45 patients developed symptoms. A subsequent study by Wilson et al studied 553 elderly patients undergoing visceral duplex ultrasonography to find 97 patients (18 %) with severe stenosis of either the celiac or superior mesenteric arteries [1]. Of the 20 patients with mesenteric artery stenosis who died during the mean 6.5 year follow-up period, no deaths were attributed to intestinal infarction. Further, of the 45 patients with mesenteric artery stenosis available for response at a mean follow-up of 6.8 years, no patient reported symptoms referable to chronic mesenteric ischemia. These two prospective studies have informed the recommendation for treatment of mesenteric artery stenosis only in symptomatic patients though the high incidence of symptoms in patients with three-vessel disease in the Thomas study may warrant further investigation of this subgroup.
Though results following treatment of CMI will be addressed separately in Chaps. 12 and 14, it is worthwhile to note that the number of in-hospital deaths associated with CMI are decreasing over time. Lo and colleagues, using the NIS, have shown a nationwide 25 % decrease in CMI-related in-hospital deaths from 1998 to 2010, from 0.16 deaths per 100,000 persons in 1998 to 0.12 deaths per 100,000 persons in 2010 [8]. Interestingly, the Centers for Disease Control Wide-ranging Online Data for Epidemiologic Research (CDC WONDER) database has demonstrated a relatively stable CMI attributable mortality ranging between 0.04 and 0.07 deaths per 100,000 persons over the same time period [8]. This discrepancy between in-hospital CMI-associated deaths as determined by the NIS and CMI attributable deaths as determined by the CDC WONDER database is likely due to the NIS inclusion of patients admitted to the hospital with CMI who go on to die of other causes (i.e., myocardial infarction or other catastrophic insult). Reconciling these two data sources, it appears that CMI-related deaths are either stable or falling in recent years. These compelling data are likely the result of multiple factors including improved medical prevention with statin agents, earlier diagnosis with the increased utilization of cross-sectional imaging, and, finally, earlier treatment with the increasing use of endovascular techniques.
Acute Mesenteric Ischemia
Epidemiology
Accounting for less than one in every 1,000 admissions [19], acute mesenteric ischemia (AMI) is a rare though highly morbid condition whereby acute onset of bowel hypoperfusion is brought on through a variety of mechanisms. In contrast to CMI, a condition for which hospitalizations have increased, hospital admissions for AMI have decreased from 9 per 100,000 US persons in 1999 to 7 per 100,000 US persons in 2010 (Fig. 4.2) [8]. Regarding the treatment of AMI, prior NIS data published by our group showed that from 1988 to 2006 the absolute number of procedures for the treatment of AMI in the United States remained largely stable at approximately 700 to 800 cases annually though the proportion of endovascular cases increased substantially [9]. Accordingly, population-adjusted data by Lo et al have shown that endovascular interventions for AMI have risen nearly threefold from 0.06 cases per 100,000 US persons in 1999 to 0.18 cases per 100,000 US persons in 2010 [8]. Yet, while AMI-related hospitalizations and AMI-related endovascular interventions appear to be trending in opposite directions, the reasons for this are likely multifactorial given the varied nature of AMI etiologies.
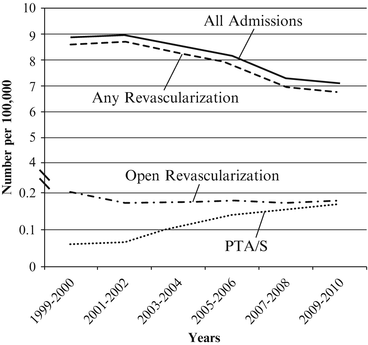

Fig. 4.2
Annual number of admissions per 100,000 persons for acute mesenteric ischemia in the Nationwide Inpatient Sample
AMI is the final common pathway for several clinical entities, each of which has its own unique epidemiology: (1) mesenteric arterial thromboembolic disease, (2) mesenteric venous thrombosis (MVT), and (3) nonocclusive mesenteric ischemia (NOMI). Acosta and colleagues reviewed 270 AMI cases detected at either autopsy or operation at a regional hospital in Sweden to find the following distribution of etiology: 67 % thromboembolic, 16 % mesenteric venous thrombosis, 15 % nonocclusive mesenteric ischemia, and 2 % indeterminate [20]. Similarly, a clinical series by Endean et al reported on 58 cases of thrombotic AMI, of which 38 % (N = 22/58) were related to arterial embolism, 36 % (N = 21/58) to arterial thrombosis, and 26 % (N = 15/58) to venous thrombosis [21]. Other review data showed a similar etiologic pattern with superior mesenteric artery (SMA) embolism accounting for approximately half of all AMI cases, followed by SMA thrombosis in a quarter of cases and nonocclusive mesenteric ischemia and mesenteric venous thrombosis in 20 and 5 % of cases, respectively [19]. As each of these varies with respect to epidemiology, they will be addressed individually in this space.
Thromboembolic
Though data on the incidence of thromboembolic specific AMI have generally been limited to individual case series, a Swedish population-based autopsy study by Acosta et al has shed considerable light on this area. In Malmo, Sweden, a community in which 87 % of deaths are investigated by autopsy, 213 cases of thromboembolic AMI were identified from over 30,000 autopsies performed from 1970 to 1982; these were then added to the number of operations performed for AMI at the lone Malmo regional hospital over a 3-year sample period to produce a thromboembolic AMI incidence of 8.6 cases per 100,000 person-years [22]. Median age of those who died from thromboembolic AMI was 81 years with two thirds of cases being female. Incidence of AMI nearly doubles with each 5-year interval above age 70 with an incidence of 25 cases per 100,000 person-years for ages 70–74 that then rises to an incidence of 217 cases per 100,000 person-years for patients greater than 85 years. Interestingly, adjustment for the greater longevity of females in this population showed that female gender was not, in fact, an independent risk factor for AMI in this cohort.
The epidemiology of thromboembolic AMI may be influenced by the prevalence of conditions that place patients at increased risk. The pathophysiology of thrombotic AMI suggests that conditions such as prior cerebrovascular accident, aortic wall thrombosis, disseminated cancer, and CMI may increase risk of thrombotic events [20]. Not surprisingly, case series on AMI have documented rates of preexisting CMI in 0–43 % of cases [20, 23–25]. Risk for embolic AMI is increased by comorbid conditions suggestive of prior embolic events such as cerebrovascular accident or predisposing to embolic events including atrial fibrillation, congestive heart failure, and recent myocardial infarction among others [26]. A report by Batellier and colleagues on 82 consecutive patients treated for SMA embolism over a 22-year period (1966–1988) demonstrated a history of atrial fibrillation in 79 % (N = 65/82) of cases and a history of prior embolic event in 35 % (N = 29/82) [25]. Accordingly, autopsy study of 122 patients with AMI attributable death showed a cardiac source of thrombus in approximately half of cases (N = 58/122) [27]. As the management of atrial fibrillation improves with better adherence to anticoagulation guidelines, it is hypothesized that the incidence of embolic AMI may also decrease though population level in this regard is lacking [26, 28]. Data from the National Health and Nutrition Examination Survey (NHANES) database supports this possibility as it demonstrates a sharp increase in the use of aspirin, clopidogrel, warfarin, and statin drugs over the period from 1999 to 2010 [8].
Mesenteric Venous Thrombosis – MVT is encountered in the acute form, which commonly presents as bowel infarction, or the chronic form, which may present more insidiously with nonspecific symptoms or esophageal varices in cases with portal vein involvement. Here we will focus on acute MVT. Again looking toward clinical and autopsy data from Malmo, Sweden, study periods from 1970 to 1982 and 2000 to 2006 showed a rising incidence of mesenteric venous thrombosis (MVT) with 2.0 cases per 100,000 person-years in the earlier period as compared to 2.7 cases per 100,000 person-years seen in the latter, this on the basis of 63 (56 % autopsy) and 51 (12 % autopsy) identified MVT cases, respectively [29, 30]. A rise in the use of abdominal imaging has certainly contributed to increased MVT detection as 69 % of patients in the period 2000–2006 were diagnosed with CT scan as compared to 0 % in the earlier period. Even within the period 2000 to 2006, a greater proportion of cases were diagnosed by CT scan in the latter half (2004–2006: N = 10/21) than in the former (2000–2003: N = 25/30); p = 0.026. With an increasing proportion of cases diagnosed by cross-sectional imaging, it is likely that these data represent an increase in detection rather than an increase in true incidence. MVT involved secondary thrombus, or those cases in which an etiologic factor has been identified, in a majority of cases (80 %). As our understanding of congenital and acquired thrombotic states improves, the number of MVT cases attributed to primary thrombus, or those of unidentified etiology, has decreased [31]. Though men and women were equally likely to develop MVT, advancing age was correlated with increased incidence of MVT with septuagenarians having over twice the risk of those aged 60–69 years, 11.3 vs. 4.8 cases per 100,000 person-years, respectively. Approximately two thirds of these patients (N = 34/51) were noted to have either a congenital or acquired thrombophilia with the remainder having either intra-abdominal infection or other pro-thrombotic conditions such as pancreatitis (18 %), post-surgical trauma (8 %), or inflammatory bowel disease (2 %). Rhee et al from the Mayo Clinic published on 72 cases of MVT (57 acute) from 1972 to 1993 with findings largely in agreement with the Swedish data [32]. Similar rates of secondary thrombus (75 %) were seen, and again thrombophilia or prior surgery were among the leading causes of MVT. CT scan was abnormal in all patients with acute MVT who were studied. Though rare, acute MVT diagnosis has increased over time owing to the improved quality and increased use of intra-abdominal vascular imaging.
Nonocclusive Mesenteric Ischemia
As the name would imply, NOMI refers to intestinal gangrene in the setting of patent mesenteric vessels [33]. First described in a 1949 case report from the Massachusetts General Hospital [34], NOMI describes a clinical syndrome comprising a number of entities which share the common pathophysiologic elements of mesenteric vasoconstriction, intestinal hypoxemia, ischemia–reperfusion injury, increased intestinal metabolic demand, and infection [35]. One in 5,000 hospital visits is attributed to NOMI [19] though given its nebulous description and frequent association with other pathologies, the true incidence may be difficult to define. Autopsy data from Sweden by Acosta and colleagues demonstrated an incidence of 2.0 cases per 100,000 person-years for fatal NOMI with incidence in octogenarians noted to increase to 40 cases per 100,000 person-years [36]. Nested case–control comparisons with non-NOMI autopsy patients identified fatal heart failure, history of atrial fibrillation, and recent surgery to be risk factors for fatal NOMI. Mesenteric stenosis also appears to have an association with NOMI as 25 of 62 patients in this series showed SMA stenosis at autopsy, with 14 of those also having celiac stenosis. Yet, importantly, the critical aspect in the development of NOMI is a low flow state, a state that may occur with or without concurrent mesenteric stenosis. Beyond these few epidemiologic studies, data on NOMI are primarily drawn from small case series and case reports relating it to varied conditions associated with either profound isolated vasoconstriction (e.g., cocaine use, digitalis toxicity) or low flow states with or without critical illness and vasopressor support [37–41].
Natural History
Though varying slightly according to etiology, the natural history of AMI, without intervention, follows an almost uniformly fatal progression from bowel infarction to sepsis and death. Even in the setting of operative intervention, outcomes remain poor. Case series prior to the endovascular era, with intervention rates ranging from 63 to 100 %, are confirmatory of this dismal prognosis with 30-day mortality rates for AMI ranging from 32 to 82 % [20, 21, 23–25, 42–45]. Contemporary series suggest that endovascular therapy may improve outcomes with a 30-day mortality rate of 24 % versus 42 % for endovascular versus open revascularization, respectively (p = 0.034) [46]. However, it must be noted that these data were retrospective and most likely confounded by disease severity as 63 % of the open group underwent bowel resection as compared to 19 % in the endovascular group. A thorough treatment of open versus endovascular treatment for AMI will be presented in Chap. 20. Similarly, NOMI has an extremely poor prognosis though this is in part related to the nature of its association with critical illness in general [35]. In contrast, MVT has a slightly better prognosis than do AMI or NOMI with published reports showing operation for bowel resection in one to two thirds of patients and overall mortality rates of approximately 20 % [20, 30, 47, 48]. On a population level, Lo and colleagues, using the NIS, have shown that in-hospital mortality for AMI regardless of etiology or treatment approach has fallen from 1999 to 2010 [8]. Open repair has shown the most dramatic improvement for in-hospital mortality rate, from 43 % to 33 % over the study period. This was followed by the change seen in in-hospital mortality rate following endovascular revascularization, which has gone from 20 % to 15 %. CDC WONDER data, also reported by Lo et al, have similarly shown a decrease in AMI-related mortality going from 1.5 deaths per 100,000 US persons to 0.6 deaths per 100,000 US persons. Given these population-level figures, it appears that the overall management of AMI including diagnosis and treatment may be improving over time.
Median Arcuate Ligament Syndrome
Epidemiology
Alternately known as celiac artery compression syndrome or Dunbar syndrome, median arcuate ligament syndrome (MALS) refers to chronic, recurrent postprandial abdominal pain due to mesenteric ischemia in the setting of celiac artery compression by the median arcuate ligament (Fig. 4.3). From an anatomic perspective, Lipshutz and colleagues, in a 1917 cadaveric study reporting anatomic variants of the celiac axis in 83 patients, were the first to describe compression of the celiac origin by the diaphragm noting that such a configuration occurred “not infrequently”[49]. Further autopsy study by Derrick et al in 1959 reported that 44 % of unselected patients demonstrated stenosis of the celiac axis origin with half of these having stenosis >50 % [50]. Dunbar and colleagues then associated this anatomic finding with symptomatology when they noted that 15 of 27 patients with an abdominal bruit and unexplained postprandial abdominal pain showed compression of the celiac trunk on angiography [51]. This study as well as others prompted a dedicated autopsy study on the relationship of the celiac axis origin to the median arcuate ligament by Lindner et al who showed that in 75 unselected cadavers, 25 had a celiac artery origin cephalad to the median arcuate ligament with this configuration being more prevalent in females than males [52]. Thus, the anatomic conditions under which this pathology occurs have been shown in as many as one third of patients in general cadaveric studies with a preponderance of females showing celiac compression.
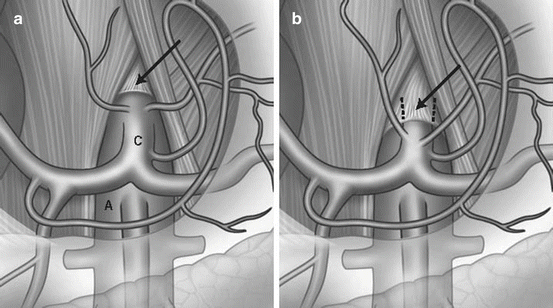

Fig. 4.3
(a) Normal relationship of celiac axis with respect to diaphragm. (b) Compression of the celiac axis by the median arcuate ligament
However, while cadaveric studies have demonstrated celiac artery compression in up to one third of patients, clinical manifestations of MALS occur much less frequently. Studies examining the correlation of clinical symptoms with anatomic findings have shown that celiac artery compression alone rarely, if ever, yields the symptoms of MALS as the gastroduodenal artery provides adequate collateral flow to the foregut. In fact, a 2001 study by Park and colleagues evaluated the celiac axis origin in 400 consecutive asymptomatic patients undergoing chemoembolization of hepatic tumors to find that 7.3 % (N = 29) of patients had celiac axis stenosis, 3.5 % (N = 16) due to median arcuate ligament compression and 2.5 % (N = 10) of indeterminate etiology [53]. Thus, it is hypothesized that MALS may relate to a neuropathic mechanism secondary to compression of the celiac plexus by the median arcuate ligament. Given that anatomic configuration alone is insufficient to cause clinical symptoms, the association of celiac artery compression and clinical symptoms has been determined by analysis of symptom relief following celiac artery decompression. Reilly et al reported long-term outcomes on 51 highly selected patients undergoing celiac artery decompression with or without celiac artery revascularization [54]. Long-term symptom relief was achieved in 53 % (N = 8/15) of patients receiving median arcuate ligament release alone and 76 % (N = 22/29) of patients receiving ligament release with accompanying revascularization. This series, one of the largest published, reinforced a female predilection (N = 39/51; 77 %) with a mean age of 47 years at time of treatment. MALS has also been reported in the pediatric population with one of the largest series showing a similar proportion of females (N = 42/46; 91 %) and similar rates of symptom relief following surgery (N = 31/46; 67 %) [55]. In summary, though population-level data on the incidence of MALS are lacking, case series suggest that MALS is a rare condition to be considered in patients, particularly females, with the appropriate symptoms and anatomy.
Natural History
The natural history of MALS is marked by diagnostic delay given that its mere existence is doubted by some and that it is a diagnosis of exclusion with nonspecific symptoms for the remainder. A contemporary series by Sultan and colleagues demonstrated a mean symptom duration of 34 months prior to diagnosis at a tertiary referral center [56]. Multiple reports have noted patients with MALS to have undergone other, unrelated abdominal procedures such as cholecystectomy, appendectomy, or others in attempts to characterize and/or alleviate abdominal pain [54, 57]. Definitive treatment is surgical, but reports of symptomatic relief have varied widely related to a heterogeneous case mix in many series with a wide variety of abdominal symptoms and often a high incidence of comorbid psychiatric illness [54, 58–64]. Results following surgical intervention will be discussed in greater detail in Chap. 32.
Isolated Mesenteric Artery Dissection
Epidemiology
Mesenteric artery dissection frequently occurs as a complication of abdominal aortic dissection; however, isolated mesenteric artery dissection (IMAD) is quite rare with only 106 total cases of isolated SMA dissection reported as of 2008 [65]. Though population-level data on the incidence of IMAD is unavailable, a flurry of case series in recent years suggests that IMAD diagnosis is increasing due to the widespread use and improving quality of abdominal imaging [66–72]. The vast majority of IMAD cases involve the SMA though reports have described the celiac or other visceral vessels in rare cases [65, 66, 72]. Demonstrating the growing need to better understand IMAD and facilitate its discussion in the literature, Sakamoto and colleagues have proposed a radiology-based, morphologic classification system on the basis of their institutional experience [73]. Pooled data on the 106 cases of isolated SMA dissection reported prior to 2008 show a mean age of 54 years and a 4:1 ratio of male-to-female patients [65]. Epidemiologic data for IMAD is lacking though certain risk factors have been suggested including hypertension, smoking, connective tissue disease, atherosclerosis, trauma, and inflammatory disorders [66]. Fourteen of 17 (82 %) of patients reported by Choi et al were smokers, while Takayama and colleagues noted 2 of 19 patients to be have connective tissue disorders (systemic sclerosis and Sjogren’s syndrome) [66, 72]. Rarer still are cases attributable to SAM as mentioned in the section on CMI. Further investigation will be required to better delineate the true incidence of IMAD and to better characterize the patients affected.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


