4 Engineering and Construction of Pacemaker and ICD Leads
Tremendous advances have occurred in implanted cardiac device therapy, from the evolution of pacemakers to the modern era of high-voltage defibrillation and resynchronization devices. Advances in the components, integrated circuitry, size, and software of the implantable pulse generator are readily apparent, but the revolutionary progress in the design and manufacture of device leads is often not appreciated. In any device system, the lead is the critical interface linking the pulse generator and the myocardium; it allows for communication of electrical signals from the heart to the pulse generator through the sensing circuitry, and in the reverse direction, it allows for bradycardia pacing or tachyarrhythmia therapy. Although leadless pacing1 and defibrillation2 device systems are being actively developed, leads clearly will remain an integral component of device therapy for the foreseeable future.
 Pacing Leads
Pacing Leads
Historical Milestones in Development
Permanently implantable pacing leads evolved from the temporary pacing wires that were first used to provide bradycardia support.3 The initial permanent transvenous leads were unipolar and consisted of a basic conductor, an insulator, and a connector pin. The electrodes were large and polished with high-polarization properties, low electrode-tissue impedance, and excessive current drain. No lumen was present in these leads to allow for stylet insertion, so lead implantation was a long, difficult task. Also, no fixation mechanism was present, so lead displacement rates were high. The development of bipolar pacing leads minimized far-field oversensing,4 requiring a major reconsideration of lead body design and structure, because two longitudinal conductors needed to coexist inside the lead, separated only by a thin layer of insulation. This and subsequent increases in complexity required advances in materials science so as not to compromise reliability.
The development of passive-fixation tines was a huge step forward and dramatically reduced the rate of reoperation for lead dislodgement compared with existing flange-tipped pacing leads.5–7 Active-fixation helices were introduced subsequently and further improved the stability of implanted leads. Modern electrodes were developed with small geometric surface area but large effective area as a result of porous surfaces, and these optimized the trade-off between high current density and low polarization. Chronic threshold rises and late exit block were still major problems in cardiac pacing at that stage, however, and remained so until the revolutionary incorporation of an elutable dexamethasone reservoir into a porous-tip electrode, essentially eliminating this complication.8 Although this likely resulted from the glucocorticoid and not the redesigned porous titanium-tip electrode, this remained unproved until a pioneering randomized double-blind trial compared two otherwise identical leads and electrodes, with and without steroid elution.9 This unequivocally demonstrated the benefit was the result of the dexamethasone. Subsequent advances have included the universal standardization of connector pins (and consequently device headers), improving lead longevity through the use of better component materials, and improving sensing through the use of narrower bipoles.
Lead Structure And Polarity
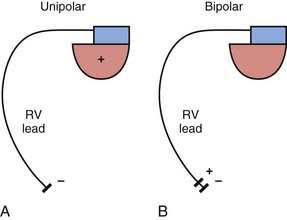
The basic functions of any cardiac device lead—pacing and sensing—require conductors for current flow between the pulse generator and myocardium and insulators to prevent short-circuiting of this current. These elements may be configured in two ways. First, a pace/sense circuit may be composed of a lead containing one conductor that connects to a negatively charged tip electrode, the cathode, from which electrons flow through the myocardium and thoracic cavity back to the active, positively charged pulse generator (IPG), the anode (Fig. 4-1, A). Such a lead, with only one conductor and electrode, is called a unipolar lead because only one electrode is in contact with the heart (although it is part of a bipolar circuit). It has been established that pacing thresholds are lower at most pulse widths when the intracardiac pacing electrode is configured as the cathode rather than as the anode10 (see Chapter 1). Although once the only option, unipolar leads have largely been replaced by bipolar leads. The alternative system consists of a lead body containing two conductors (separated and surrounded by insulation) that connect to a cathode-tip electrode and an anode ring electrode located several millimeters more proximally (Fig. 4-1, B). The IPG is not an active electrode in a bipolar circuit, and such leads are said to have bipolar configuration.
Unipolar systems have a large interelectrode distance from the tip of the intracardiac lead to the IPG, and this large field of view makes them vulnerable to the oversensing of myocardial signals (T waves or electrograms from another chamber, known as “crosstalk”) and far-field nonmyocardial signals such as pacing artifacts (another form of crosstalk), skeletal myopotentials, and nonphysiologic electrical “noise” in up to 38% of patients.11 Electrical signal oversensing can have significant consequences on pacing behavior, including ventricular output inhibition and inappropriate mode switching caused by far-field ventricular oversensing on the atrial channel.12,13 Because of the additional consequence of inappropriate shock delivery if oversensing were to occur in an ICD, it is particularly important for ICD leads to have bipolar sensing circuitry. Since the IPG itself serves as one of the electrodes in a unipolar system, skeletal muscle capture and pectoral muscle stimulation may result in the setting of a higher programmed pacing output. Bipolar leads have been shown to have similar but slightly higher, acute and chronic stimulation thresholds to unipolar leads.4 Bipolar leads also carry an element of redundancy in that they can be used in either the bipolar or the unipolar configuration, as in isolated failure of the proximal, anodal conductor, with no demonstrated difference in performance between the two pacing configurations.14 In addition, the much lower amplitude of the bipolar pacing artifact greatly reduces the likelihood of crosstalk and oversensing. The great advantage of bipolar leads is related to their superior sensing properties. These factors have led to the near-universal adoption of the bipolar lead as the configuration of choice in cardiac device systems.15 The only potential disadvantage of bipolar leads is that reliability is lower than with the less complicated unipolar lead,16 although some studies have not supported this contention.15,17
However, insulation failure usually has negligible effects on unipolar lead performance, a clear reliability advantage. In terms of the structure of the lead body, the simple, single-conductor design of unipolar leads had to be modified to accommodate the greater number of components and increased complexity of the bipolar lead body. There are two main bipolar lead designs, coaxial and coradial (Fig 4-2). Coaxial leads have an inner conductor that extends down the length of the lead to the tip electrode, the cathode, arranged in a coil configuration with a central lumen to allow for passage of a stylet at implantation. This coil is covered by a cylindrical length of inner insulation, which in turn is wrapped by another coil conductor that also runs down the lead to the ring electrode, the anode. A second, outer layer of insulation and lead covering protect the ring conductor from the outside environment, thereby completing the design. Although this coaxial model has been the industry standard for pacing leads for many years, the resulting bulk and stiffness of this four-layer design are significant compared with the simpler unipolar leads. Coradial bipolar leads addressed some of these concerns with new conductor and insulator technology that was subsequently used in ICD lead design. In coradial leads, a single coil extends down the length of the lead (again with a central lumen to allow for stylet insertion) and consists of two parallel, alternating conductor strands, one of which connects to the cathode and the other to the anode. Each conductor strand is individually coated with a bonded layer of ethylene tetrafluoroethylene (ETFE) fluoropolymer insulation (originally by DuPont, Wilmington, Del), which serves to insulate each strand from the other, despite being intertwined. The single, two-component coil is surrounded by a single, outer insulation covering.18 These leads have comparable bulk (~5F diameter) and flexibility to unipolar leads, often with tines or a fixed, nonretractable helix to minimize size; electrical parameters and reliability have not been demonstrably inferior to coaxial leads.18–21
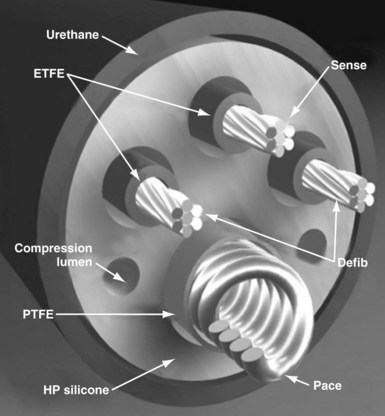
Defibrillator leads have even greater complexity, with two, three, or four conductors that connect to the pacing/sensing electrodes as well as to the high-voltage shocking coil(s), depending on the specific type of lead. Because of the prohibitive bulk that would result from a coaxial design with more than two conductors, ICD leads generally have a different type of structure, known as a multilumen design. This type of ICD lead consists of a long cylinder of insulating material, with separate internal channels running down its length (Fig. 4-3). Each conductor runs down an individual channel, usually with its own additional covering tube of insulation. The configuration and number of channels differ, depending on the number of conductors, the specific manufacturer, and whether air-filled channels are also incorporated into the design to improve lead handling and protect the conductors from flexion-related damage in the dynamic intracardiac and intravascular environment.
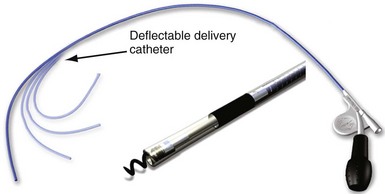
One other lead body structure worth mentioning are small (4.1F diameter), isodiametric, lumenless pacing leads (Model 3830 SelectSecure; Fig. 4-4). The lack of a central lumen and the use of a conductor cable (rather than coil) allows for increased insulation redundancy, high tensile strength, and reduced bulk. It also means that the active-fixation helix is nonextendable because there is no central coil conductor to transmit torque from the connector pin to extend or retract the helix. Fixation of such leads to the myocardium is achieved by turning the entire lead body. To enable implantation without a stylet, the lead is deployed through an 8.5F steerable catheter delivery system (SelectSite C304). Short-term results from this lead have been reasonable, with no signs of increased lead displacement or fracture risk.22,23 The small external diameter of this lead has been considered to be of some benefit in pediatric patients24 and patients with congenital heart disease,25 but the suggestion of a resulting improvement in chronic venous patency from the smaller size is yet to be substantiated in long-term follow-up. In addition, selective site pacing, in both the atria and the ventricles, is facilitated by the deflectable sheath, although steerable and preformed stylets can also be used with conventional leads to achieve this. No published experience with extraction of chronically placed lumenless leads exists, and the lack of a lumen to accommodate a lead-locking device may be a significant disadvantage of this design.
Conductors
Both the coaxial and the coradial bipolar pacing lead body designs have been in widespread use and proved to be relatively fracture resistant over time. This durability is at least partially related to the materials and design of the conductor elements used in pacing leads. One study found conductor fracture over a 17-year follow-up in 19 of 561 right ventricular pacing leads (3.4%) and then generally at points of high stress, such as at the lead anchoring sleeve and the costoclavicular ligament (subclavian crush).26 The basic material used for most conductors over the last 3 to 4 decades has been MP-35N (SPS Technologies, Cleveland), an alloy of nickel, cobalt, chromium, and molybdenum. The main advantage of MP-35N is its high strength and resistance to corrosion.27 Its main disadvantage is its high electrical resistance, but this has been overcome with the development of composite-wire conductors that incorporate low-resistance metals such as silver and stainless steel with high-strength materials such as titanium, platinum, and platinum-iridium alloy.
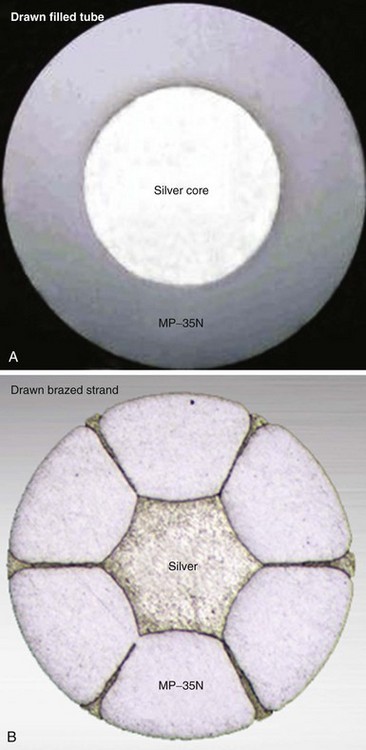
In pacing leads, these materials are generally incorporated into a drawn filled tube (DFT) composite-wire conductor strand consisting of a thick, strong body of MP-35N or titanium that has been filled with a central core of softer, low-resistance metal such as silver, often encased in a further outer shell of platinum alloy (Fig. 4-5, A). The DFT conductor strands are then formed into multifilar (i.e., multiple strands or filaments) coils with a central lumen in coaxial leads, as described earlier, or further coated in a layer of ETFE fluoropolymer before being arranged in bifilar coradial coils,28 again with a central lumen. The alternative way of forming a composite-wire conductor is the drawn brazed strand (DBS) method, generally consisting of six strands of a high-resistance material (e.g., MP-35N) that are tightly molded over a central strand of silver, such that the inner, low-resistance metal is forced between and around the strong outer strands (Fig. 4-5, B). This results in a low-resistance, durable composite conductor wire that can once again be formed into a multifilar coil for use in pacing leads.29 However, it is also used to form strong, multifilar, braided cables that are employed as the conductors for the high-voltage circuitry of ICD leads. DBS cables are discussed later.
Insulation
Pacing lead insulation is a critical and vulnerable component that is often the cause of lead failure.30,31 One of the problems with the major materials used for lead insulation (polyurethane, silicone rubber, fluoropolymers) is that they were not originally designed specifically for this purpose, and each has disadvantages when used as part of a biologic pacing system. They were incorporated by early lead engineers into their designs for lack of any purpose-built alternatives, and it is only recently that any viable, tailored alternatives have been developed.
Poly(ether)urethane is a synthetic, segmented polymer with very high tensile strength and resistance to mechanical abrasion. From a physical perspective, this means that a thinner layer of insulation can be used to cover the lead conductors, thereby reducing the overall lead diameter. Also, polyurethane has excellent lubricity and handling characteristics, with a low frictional coefficient, which can facilitate implantation of two or three lead systems by decreasing the physical interactions between the leads. However, polyurethane leads are stiffer and not fully biostable, being subject to in vivo polymer degradation that can cause insulation and late lead failure.32 The two main types of polyurethane are Pellethane 80A and Pellethane 55D (Upjohn, CPR Division, Torrance, Calif), with 80A particularly prone to biodegradation.
Two mechanisms of in vivo, biologically mediated degradation of polyurethane predominate: environmental stress cracking (ESC) and metal ion oxidation (MIO)33 (Fig. 4-6). When polyurethane is subjected to repeated mechanical stresses in the presence of surface-active agents provided by macrophages and α2-macroglobulin, parallel polymer molecules are disordered and interchain bonds weakened. This is the mechanism of ESC,34 manifesting as a deep surface cracking visible with electron microscopy, particularly at the anchoring sleeve and venous entry portions of the lead. By comparison, no mechanical stress is required for MIO to develop because this is a chemical reaction that occurs particularly at the conductor-insulator interface.35 Biologic oxidants, such as peroxide from surrounding inflammatory cells,36 can result in the release of metal cations from the conductor, especially cobalt and nickel ions. These cations cause oxidation of the soft zones in the polymer, starting at the α-carbon of the ether bond and resulting in chain break. The harder Pellethane 55D is much less susceptible to both ESC and MIO than 80A but is significantly stiffer. Nevertheless, the long-term performance of Pellethane 55D has been much better than Pellethane 80A, a material notoriously prone to failure.

Figure 4-6 Mechanisms of polyurethane insulation failure on electron microscopy.
(Courtesy St Jude Medical, Sylmar, Calif.)
Silicone rubber (polysiloxane) has none of the disadvantages of polyurethane. It is completely inert and biostable over extended periods and thus not vulnerable to MIO or ESC. Silicone is more flexible, which may decrease the risk of damage to cardiac structures, including lead perforation. It is also more thermally resistant to the effects of electrocautery, which can be a significant advantage during pulse generator replacement or system revision.37 Initially, a softer peroxide-catalyzed silicone rubber, MDX4-4515-50A, was used for pacing leads, but new versions with higher tensile strength and abrasion resistance have been developed. These include high-performance (HP) silicone, extra-tear-resistant (ETR) silicone, and Novus (Med-4719, Nusil Technologies, Carpinteria, Calif), produced by hybridizing HP and MDX4 silicone. Unfortunately, no variety of silicone rubber has the positive characteristics of polyurethane, including surface lubriciousness and ease of handling. Although these friction-related problems of silicone can be addressed by an outer layering of polyurethane, the main problem with silicone relates to its lower tensile strength and susceptibility to abrasion and tears (Fig. 4-7, A). This means that silicone insulation is more prone to damage during implantation and subsequent interactions in the device pocket, and that a thicker insulation layer must be used to maintain lead reliability, thereby increasing lead bulk. Also, as with glass, silicone is a fluid material and thus prone to a gradual flowing process away from pressure points, known as “cold flow,” which can lead to thinned, denuded, and abraded troughs or depressions adjacent to areas of thickened rubber polymer32 (Fig. 4-7, B).
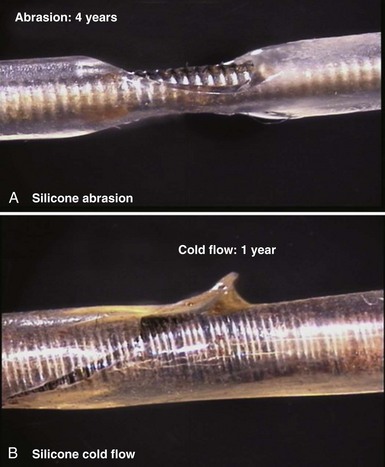
Figure 4-7 Mechanisms of silicone rubber insulation failure.
(Courtesy St Jude Medical, Sylmar, Calif.)
The fluoropolymers polytetrafluoroethylene (PTFE) and ETFE (DuPont) have some of the advantages of both silicone and polyurethane, with good abrasion resistance and biostability.38 Unfortunately, their great stiffness excludes them from functioning as the primary insulating material in any lead, but they can be used, as outlined earlier, as a thin coat on conductor strands, particularly in coradial leads. This inner insulating layer can prevent electrical communication between conductor strands and can also protect them from interacting with an adjacent outer layer of polyurethane, thereby reducing MIO.
Recently, the development of a specific insulation material for cardiac leads has suggested that the deficiencies of the standard insulators in pacing leads may finally be addressed. The material is a copolymer hybrid composed of 48% silicone, 40% polyurethane hard segment, and 12% polyhexamethylene oxide soft segment; it is called Elast-Eon (Aortech Biomaterials, Clayton, Victoria, Australia). This blending of insulating materials results in the product having the strength, lubricity and abrasion resistance of polyurethane, but the flexibility and biostability of silicone.39 Early bench and clinical testing have been promising to date, with little evidence of cold flow, ESC, or MIO phenomena, the main weaknesses of silicone and polyurethane. The material has been used in the St. Jude Medical Optim family of pacing (Models 1888T and 2088T) and ICD leads (Durata), but no long-term data on its chronic performance are yet available.
Electrodes
The materials used for lead electrodes have evolved over the past 40 years and have included titanium, anodized platinum, platinum-iridium alloys, Elgiloy (an alloy of cobalt, iron, chromium, molybdenum, nickel, and manganese), and vitreous carbon. All these display relative inertness in vivo, which minimizes inflammatory reactions and corrosion over the long term. Minor corrosion is seen, however, with Elgiloy and platinum alloys when in situ for extended periods, although this is of uncertain clinical significance.40 While attempting to control for other differences, several human and animal studies have compared these materials, but the results are conflicting.41–44 In general, applications have been found for these materials in electrode composition, with most having reasonable in vivo performance and longevity, although vitreous carbon cathodes have particular strength, inertness, and long-term electrical reliability.45
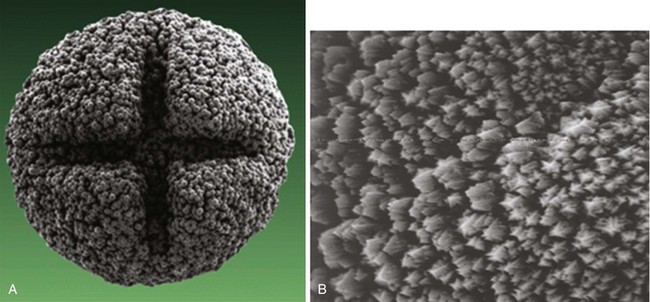
More important than the precise material composition of electrodes seems to be their geometry, particularly the surface area. A large cathodal surface area in contact with the endocardium results in a low current density at the electrode-tissue interface and consequently a higher capture threshold. As tip electrodes were reduced in surface area, apart from better thresholds, higher tissue-electrode impedances were also seen, and this increased pulse generator longevity.46,47 Against this beneficial effect of a smaller electrode is the adverse effect on tissue polarization seen with a smaller electrode surface area. Polarization refers to the electrical afterpotential generated at the electrode-tissue interface by ionic movement induced by the pacing stimulus. At pacing pulse delivery, a layer of positively charged cations is attracted to and surrounds the negatively charged cathode (and a larger layer of negatively charged anions surrounds the cation layer), which opposes current flow from the pacing electrode. This polarization potential is recorded as an afterpotential following the stimulus artifact. To maximize the advantages of small-radius electrodes, yet minimize the disadvantage of the polarization effect more prominent with smaller electrode surface area, current electrodes are small in size but have a complex, porous microscopic surface (Fig. 4-8). Regardless of the method of achieving a complex, highly textured surface, such as coating the electrode with microspheres or a woven mesh of microscopic metallic fibers, the resulting large surface area has proved beneficial with regard to pacing and sensing performance.

Figure 4-8 Sintered porous electrode (left) and laser porous electrode (right).
(From Hirshorn MS, Holley LK, Skalsky M, et al: Characteristics of advanced porous and textured surface pacemaker electrodes. PACE 6:525-536, 1983.)
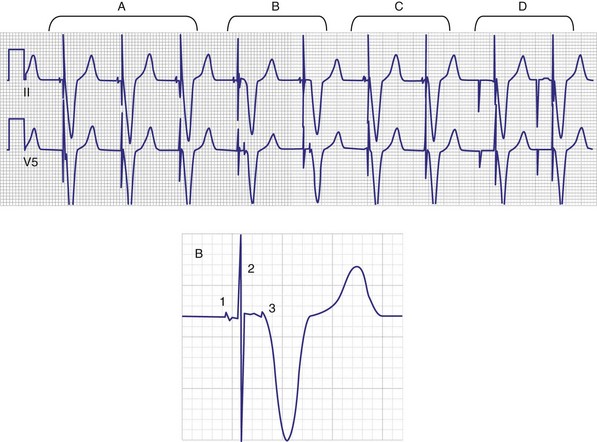
Titanium nitride–coated or iridium-coated cathodes with fractal surface structure achieve the goal of enhanced surface area with a negligible polarization signal48 (Fig. 4-9). Such electrodes have permitted the development of beat-by-beat automatic capture threshold and pacing output management systems that measure the evoked myocardial response following every pacing stimulus. By verifying myocardial capture for each paced beat, with the immediate delivery of a backup higher-output pulse if capture is lost (Fig. 4-10), the pacemaker can maintain a pacing output only marginally above the capture threshold, thereby prolonging pulse generator battery life.49 Another type of automatic threshold algorithm does not verify beat-to-beat capture, but performs an automatic threshold test daily and adjusts the pacing output accordingly (with larger safety margin above capture threshold). These types of automatic capture-detection algorithms can be unreliable in the setting of a significant polarization afterpotential, because a large polarization signal cannot be distinguished from the local myocardial evoked response.50
Electrodes implanted in myocardium elicit a strong foreign body granulomatous reaction that results in the formation of a local fibrous capsule at the lead tip, which can have a profound effect on electrical performance. Smooth-surfaced electrodes elicit the formation of a thick, electrically insulating capsule of fibrous and granulation tissue that may impede electrode fixation to the endocardium.51 Complex, porous electrodes, in contrast, are manufactured to have extremely small (20-50 µ in diameter) surface pores that allow for endocardial attachment by way of tissue ingrowth into these regions, which yields a mechanical advantage in addition to the electrical benefits mentioned earlier. A fibrous reaction also occurs with highly textured electrodes, but a lesser insulating effect is observed because the electrically active myocardium is closer to the electrode,51 resulting in improved electrical parameters over time.52
To curtail further the fibrous reaction elicited by the electrode and to decrease the incidence and magnitude of chronic threshold rises, a pharmacologic anti-inflammatory strategy was pursued with glucocorticoid-eluting porous electrodes (Fig. 4-11). These leads showed excellent long-term capture and sensing parameters, with a dramatic reduction in late exit block (i.e., loss of myocardial capture).8 In an elegant and seminal double-blind randomized trial, Mond et al.9 conclusively proved the beneficial effect of steroid elution by comparing two otherwise identical porous titanium electrodes, only one of which incorporated dexamethasone in its tip. There was no difference in capture threshold seen at implant. Although there was an expected late rise in threshold in the standard leads starting at 2 weeks, this was completely abolished in the steroid-eluting lead, both during the trial period and in long-term follow-up.53 Animal studies confirmed that the biologic basis of this electrophysiologic effect was through the glucocorticoid-induced attenuation of the inflammatory reaction, with thinner, less cellular fibrous capsules.54
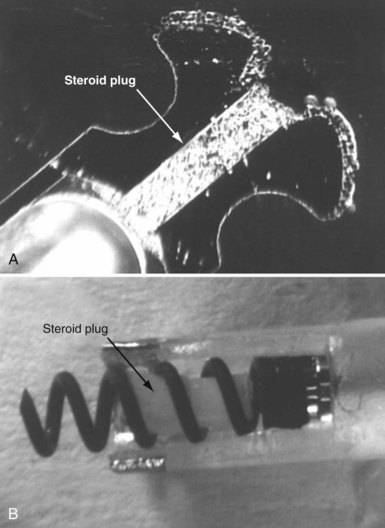
Figure 4-11 Dexamethasone-eluting reservoirs in passive-fixation (A) and active-fixation (B) leads.
(Courtesy St Jude Medical, Sylmar, Calif.)
In bipolar leads, another factor affecting electrical performance, particularly far-field sensing, is the interelectrode distance between the tip cathode and the ring anode. Narrow-spaced bipolar electrodes in atrial leads have been shown to reduce far-field ventricular electrogram sensing without compromising atrial sensing.55 In a randomized trial that compared an atrial lead with a 1.1-mm tip-to-ring spacing (Model 1699T Optisense, St. Jude Medical) with a standard 10-mm spacing, the close-spaced bipole showed minimal far-field ventricular sensing at a sensitivity of 0.3 mV, compared to 30% 1-year incidence in the standard-bipole group.56 This translated into a large reduction in inappropriate mode switch events, from 23% in the control group to 4% in the narrow-bipole group.
Fixation Mechanisms
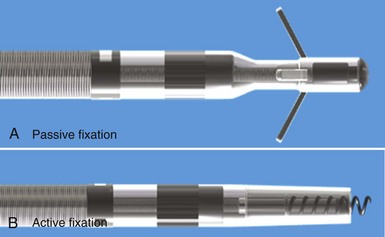
Initial pacemaker leads had no fixation mechanisms, so lead dislodgement rates were high. The first solutions to this problem were wedge-shaped flanges and fins, but these had limitations. The development of scalloped tines was the first robust solution to the problem of lead displacement,5 and comparisons with earlier methods of passive fixation were favorable.57 Tines are usually constructed from the same material as the covering insulation of the lead, typically silicone rubber, and protrude backward from the base of the tip electrode. Their orientation is designed to allow advancement of the lead for initial implantation but to prevent retraction and dislodgement by engaging the myocardial trabeculae of the right atrial appendage and right ventricular apex (Fig. 4-12, A).
Although passive-fixation tined leads perform well, with low rates of dislodgement, they are less reliable for pacing at nontraditional sites, and the development of active-fixation leads with a helix at the tip improved the options for pacing site selection (Fig. 4-12, B). These leads generally have an extendable-retractable screw made of platinum-iridium or similar alloy that is usually electrically active as part of the cathode. This terminal helix is deployed (i.e., extended out of lead) with clockwise rotation of the connector pin, which transmits torque via the central coil conductor to the helix mechanism at the other end of the lead. A small number of leads use a stylet-driven extension-retraction mechanism. Some smaller-diameter leads have a fixed helix that requires rotation of the entire lead body around the stylet for deployment into the myocardium. Steroid elution from a reservoir at the base of the helix helps to dampen the inflammatory reaction produced by traumatic engagement of the myocardium by the screw. Active- and passive-fixation leads perform similarly overall,58 with some differences related to their construction. Active leads tend to be easier to extract, whereas passive leads tend to have lower chronic thresholds with higher impedances that prolong pulse generator longevity. The clinician must always consider the potential for perforation59 and trauma to surrounding structures60 when using active-fixation leads.61
Lead Anchoring Sleeves
Securing the lead body to the fascia or muscle at the floor of the device pocket at implantation is an important step to prevent lead dislodgement. The sutures used must be fastened firmly but, given the relative fragility of lead insulation as previously outlined, must not be allowed to compromise the lead structure or integrity (Fig. 4-13). Although the anchoring sleeve provides a mechanism to achieve this balance, there has been little development in this area. After being tied down tightly, sleeves made of MDX silicone were found to produce much lower lead deformation than ETR silicone,62 but no long-term in vivo data are available. Suture sleeves that grasp the lead by snapping into place (rather than relying on circumferential deformation of sleeve by encircling suture) have been proposed but are not in current use.