Fig. 8.1
Laser (a) and RFA (b) probes. Note the larger size of the RFA probe and the 7 cm working end
Other advantages of EVLA include ease of use, speed of pull back, and decreased cost of the disposables. Because of the focused nature of the laser beam and higher temperatures, EVLA unlike RFA is associated with higher vein wall perforations. This is one of the reasons why EVLA is associated with more treatment-related pain and induration during the patient recovery phase, compared to RFA [1]. Postoperative complications such as bruising and pain were significantly less with RFA ablation with second-generation catheters than ELT in two RCT trials [5].
RFA produces uniform heating of only the endothelium. It is unlikely to produce perforation of the vein. Gibson et al. have expressed identical views in the choice between RFA and Laser [6].
Endovenous Laser Ablation
Endovenous laser ablation (EVLA) was first described by Carlos Bone in 1999. The procedure was then published by Navarro et al. [7].
The Mechanism of Action
Although the exact mechanism is not known, the widely accepted view is that endovenous laser produces a nonthrombotic occlusion of the vein by the delivery of laser energy. Heat bubbles are produced in the endothelial cells that eventually lead to their damage. Once destroyed the vessel contracts, fibrosis follows, leaving behind a cordlike structure [7, 8].
Physical Properties
Monochromatic (single wavelength) light emitted from both diode and neodymium-doped yttrium aluminum garnet [Nd:YAG] are used for EVLA [9]. Lasers used for EVLA work in the infrared wavelengths. Lasers of wavelength ranging from 810 to 1,470 nm have been used [8–10]. All wavelengths of EVLT are safe and effective. There are conflicting reports of the action of the lasers based on the different wavelengths. Studies comparing lasers of 940 and 1,320 nm have shown significantly less pain and bruising in the longer wavelength group [11, 12]. The main chromophore of 1,320 nm laser is water, while other wavelengths used for EVLA primarily target hemoglobin [11]. In our center, we have used a diode laser with a wavelength of 980 nm (Fig. 8.2).
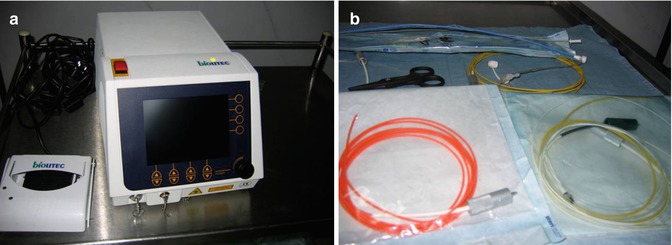

Fig. 8.2
Endovenous laser ablation equipment. (a) Laser generator and foot pedal. (b) Laser fibers
The “dose” of laser energy delivered can be expressed as joules (J)/cm of vein. A minimum laser energy of 60 J/cm is required to achieve optimum ablation [13]. The energy so delivered depends upon the diameter of the vein. A vein with a larger diameter requires more energy to be closed [14, 15]. It is pointed out that high power along with a short application time tends to have an evaporating effect, and low power along with a longer application time has a coagulating and shrinking effect [14].
Most surgeons prefer to set the power between 12 and 15 W; higher power is required when the diameter of the vein increases. A withdrawal rate of 2 mm/s at 14 W delivers 70 J/cm [13]. The laser delivery is by pulsed or continuous pullback [14]. The laser unit can give feedback regarding the dosage of energy delivered.
The laser energy is commonly delivered endovenously. But for non-cannulable veins, it can be delivered by perivenous or transcutaneous methods.
Indications and Contraindications for EVLA
Any vein that is sufficiently large (3 mm or more), relatively straight, and which can be pushed down after tumescence to a depth of 1 cm below the skin surface (to minimize risk of skin burns) is amenable to endovenous laser ablation.
The veins treated by endovenous laser include GSV and its tributaries, including incompetent anterior accessory GSV of thigh and leg, SSV and its tributaries. The inclusion criteria for treating such veins would be the presence of superficial venous insufficiency only, duplex scan reflux of over 0.5 s, patent deep venous system, cannulable veins, and ambulant patient [9, 14, 16].
EVLA is contraindicated in AV malformation, obstructive pathology of the deep veins, and patients with restricted mobility. Relative contraindications include deep vein reflux, prior treatment for varices, extremely tortuous veins, large caliber veins, patients on oral anticoagulant and hormone replacement therapy, and aneurysmal veins segments [8, 9, 16].
Technique
Preoperative Assessment
A thorough patient history is recorded and an informed consent is obtained. The high expectation of the patient regarding the outcome of the surgery needs to be tempered. The possibility of recurrence and other complications should be informed.
The limb to be treated is mapped using a 7.5 MHz Doppler probe. Deep vein is assessed to rule out deep vein incompetence or obstruction.
Anesthesia
Local anesthesia with instillation of tumescence in the perivenous plane is preferred for this procedure.
An occasional patient may need sedation or regional block (femoral nerve block). In very apprehensive patients, general anesthesia or regional anesthesia may be appropriate [17].
EVLA is an office procedure. Many of our patients prefer an overnight stay post procedure.
Tumescence
The term literally means “a swollen condition” or a “swollen part of an organ.”
Tumescent saline – Depending on the requirement, a tumescent anesthetic contains a 5–20-fold dilution of local anesthetic (15 ml of xylocaine or 15 ml of bupivacaine), epinephrine 1:100,000, with sodium bicarbonate (NaHCO3) (10 mEq/l) and diluted with physiological saline solution [18].
The tumescent saline is injected in the perivenous plane, deep to the fascia covering GSV, under ultrasound guidance along the entire length of the vein to be treated. The injection can be made by multiple punctures or by pressure flow apparatus. We prefer the multiple puncture using a long 23G spinal needle inserted into the perivenous plane under USG guidance (Fig. 8.3).

Fig. 8.3
Instillation of tumescence in the saphenous compartment. Note the proximal vein collapsed and approximated to the laser fiber
Benefits of Tumesence. Tumescent saline has the following beneficial effects [14]:
1.
It acts as a heat sump to absorb the heat escaping from the treated vein and thus protects the overlying skin from thermal injury.
2.
It compresses the vein in its circumference and gives better proximity of endothelium with the laser tip.
3.
It pushes the vein away from the skin.
4.
Local anesthetic effect.
EVLA: Steps
All the routine precautions for the use of laser fibers are to be observed. The procedure is done under US guidance. The procedure starts with painting and draping the affected limb. A local block is given at the site of cannulation of the vein, in the lower part of the thigh, or the just below the knee. Here the saphenous nerve is away from the vein. A second more proximal site should be identified and kept in reserve in the event of difficult cannulation. In case of difficulty in identifying the vein, the medial border of the shin of the tibia is identified and the vein is then located about 1 cm medial to this. The GSV is cannulated using a 16G cannula. A guide wire is then passed through the cannula and positioned so that the tip of the guide wire is at or just across the SF junction (Fig. 8.4).
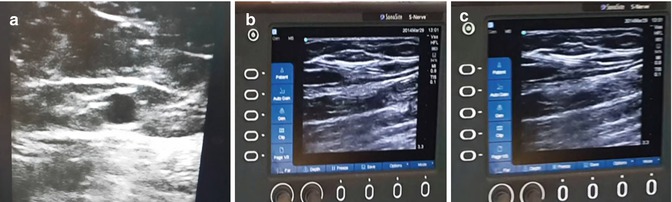

Fig. 8.4
(a) Identification of GSV – Egyptian eye. (b) Cannulation under guidance. (c) Guide wire inside GSV
A large 8 F cannula with a two-way stopcock is passed over the guide wire after dilatation of the entry point. Laser probe with its covering sheath is passed through the cannula. The bare tip of the laser fiber is kept about 1 cm outside the covering sheath so that the sheath does not get inadvertently burnt during pull back.
Diode laser of 980 nm is used. The tip of the laser fiber emits a red color of the 980 nm wavelength which is visible through the skin (Fig. 8.5).
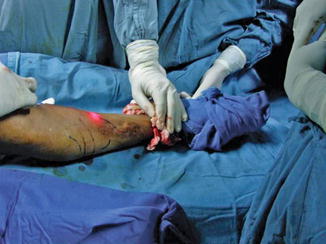

Fig. 8.5
Laser tip emitting a red glow
The laser tip is positioned about 1 cm proximal to the SF junction using the USG probe [10, 13]. Care should be taken that this is proximal to the first tributary of the GSV – the superficial epigastric vein [16] (Fig. 8.6a). Tumescent saline is injected in the perivenous plane.
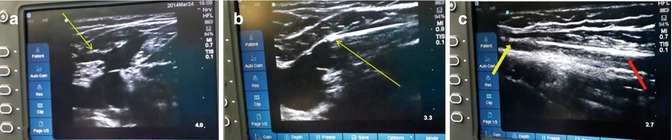

Fig. 8.6
(a) Superficial epigastric vein. Landmark for positioning tip of fiber. (b) Laser fiber tip at optimal site. (c) Yellow arrow showing treated collapsed segment of vein and red arrow the untreated segment during pull back
A Trendelenburg tilt is given to empty the veins. The vein is then treated by activating the laser generator. We use the pulse mode pullback with one pulse lasting 1 s given at each point. The power is kept between 12 and 15 W depending on the size of the vein. If the GSV is more than 1 cm in diameter, higher wattages are given.
The entire length of the vein from the SFJ to the point of cannulation is treated by a “pullback technique” with the fiber being pulled backward from the SF junction by 2 mm after each 1 s pulse (Fig. 8.6). Below-knee segment is usually not treated to avoid injury to the saphenous nerve.
A check completion duplex scan is always undertaken to confirm closure of GSV.
The telangiectatic veins are treated by transdermal ablation of the veins as most often these veins are too small to be cannulated. Thigh-length compression hosiery is applied. Patient is encouraged to walk at the earliest. Patients are discharged same day or the next day morning. Duration of postoperative compression is for 7–10 days in C2 class patients. Normal activity is encouraged soon after the procedure.
Perforators can also be treated by the technique of percutaneous ablation of perforating veins (PAP). This is a day case procedure performed under local anesthesia. Under US guidance, the perforators are cannulated and the catheter is placed at or just below the deep fascia. The veins can be ablated using laser, RFA, or sclerotherapy. Newer RFA and laser fibers are available for this purpose.
Technical Difficulties and Complications
Technical difficulties one may encounter during EVLA are difficulty in cannulation, difficulty in advancement of the fiber, and equipment failures.
Complications may be classified as early and late.
Intraoperatively patient may experience syncopal attacks, saphenous neuralgia, and sensation of transient heat due to inadequate tumescence. In the immediate postoperative period, patient may experience pain, paresthesia, bruising, skin burns, superficial vein thrombosis (SVT), and DVT [8, 14, 16]. The reported incidence of DVT in various published data varies from 0.2 to 2.2 % [14]. Most other complications are mild and only require supportive care.
Late complication is recurrence. Patients who develop SVT due to inadequate obliteration can develop recurrence due to recanalization. Three patterns of recurrence – types I, II, and III are described for RFA. This will be considered later.
Outcome of Treatment
EVLA has a high success rate. In the first three months, the ablation rate is almost 100 % [9, 10]. But with time, the success rate drops. Serial duplex scan between 1 and 3 years follow-up have shown truncal vein ablation rates of 93–99 % [16] (Table 8.1). The lower success rate has been attributed to the following:
Inadequate treatment
Failure to address the tributaries at the SFJ
Lower power used to ablate the vein
From 2004 May to 2009 May, a total number of 343 limbs were treated in our center with EVLA. Our ablation rate at the end of 36 months was 89.6 %. The complications observed include superficial thrombophlebitis 8/343, cordlike feel in the leg, infected thrombus in the treated vein segment in three patients, one case of DVT, one case where a small segment of covering sheath left behind due to fiber being pulled inadvertently and burning the covering sheath. Forty-four patients had minor skin burns and 36 recurrences at 3-year follow-up.
Table 8.1
EVLA comparison of different series
Author | Number of veins treated | Follow-up (months) | Successful ablation (%) |
|---|---|---|---|
Navarro | 40 | 4.2 | 100 |
Chang and Chua | 252 | 19 | 96.8 |
Timperman et al. | 111 | 7 | 77.5 |
Huang et al. | 230 | 6 | 100 |
Almeida and Raines | 819 | 5.3 | 98.3 |
Disselhof | 93 | 29 | 84 |
Min et al. | 121 | 24 | 93.4 |
Meyers et al. | 404 | 36 | 80 |
Neovascularization is uncommon following EVLA. This is because there is no groin dissection and specifically the superficial epigastric vein is preserved [16].
The procedure can be combined with other surgical interventions. EVLA has been performed with ambulatory phlebectomy [19]. Combined EVLA and endovenous iliac vein stenting has been reported by Raju et al. [20]. The procedure can be extended to treat patient with more advanced CEAP classes also.
Recurrence rate at 1 year following EVLA is much lower than the 10–20 % figures following HL/S. In an analysis of 280 patients comparing EVLA with conventional surgery of high ligation and stripping (HL/S), Carradice et al. reported that EVLA had lower rates of clinical recurrence compared to HL/S in short term [21].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


