Endovascular Treatment of Tibial Vessels
Charlie C. Cheng
Faisal Z. Cheema
Grant T. Fankhauser
Michael B. Silva Jr.
Introduction
The battle of endovascular therapy versus open surgical therapy for the treatment of pathologic processes in all vascular beds continues, but we are beginning to see the end. We have moved from the philosophy that only open revascularization provides effective and durable results to the growing consensus that more minimally invasive endovascular procedures can be performed safely, with reduced risk and with a level of effective clinical improvement and durability that is acceptable. Continued success requires close follow-up and repeat intervention judiciously applied when clinically warranted. In our practice and many others, open surgical procedures are reserved for failures of endovascular therapy.
Intermittent Claudication
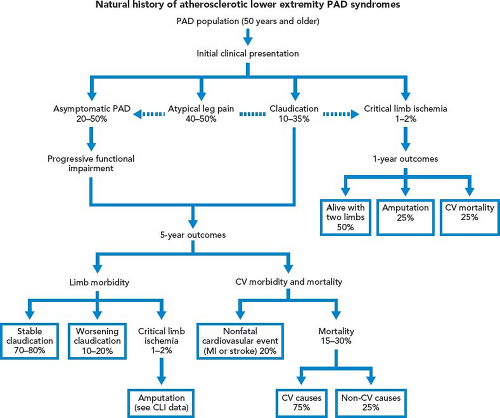
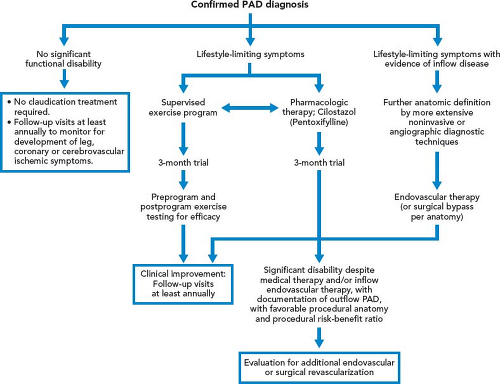
Patients with intermittent claudication (IC) are treated by risk factor modification and optimal medical therapy that include antiplatelet and statin therapy. A trial of cilostazol and supervised exercise is recommended. These therapies have been shown to improve walking distance. Patients are reassured that they are at limited risk of limb loss, approximately 2% to 3% at 5 years (Fig. 39.1). However, 25% of IC patients will see deterioration in their clinical course, usually during the first year after diagnosis; the best predictor of this decline is the initial ABI. Patients with an initial ABI of less than 0.50 have a hazard ratio of more than 2 compared with patients with an ABI higher than 0.50. Patients who present initially with low ankle pressures or patients who return with unabated, severe, lifestyle-limiting symptoms that have not adequately responded to nonoperative measures are considered for intervention (Fig. 39.2).
Critical Limb Ischemia
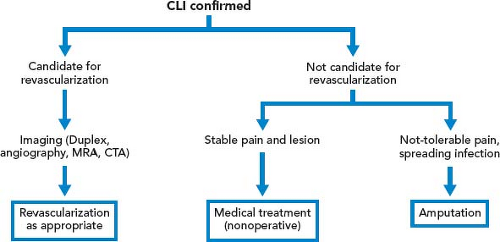
Patient who present initially with rest pain, or who progress from claudication to rest pain, is associated with a significant risk of limb loss without intervention. Similarly, patients who present with nonhealing wounds of the feet, dry gangrene, or necrotizing infection require an expeditious workup to plan a revascularization that will reestablish in-line blood flow to the foot. In case of tissue loss with infection, an immediate decision regarding the need for operative debridement or amputation prior to revascularization must be made. In case of severe sepsis with hemodynamic instability or evidence of multisystem organ failure, patients may require amputation prior to revascularization. However, if a patient with systemic toxicity from the infection responds rapidly to administration of IV antibiotics, revascularization prior to debridement may minimize tissue loss (Fig. 39.3).
Diabetic Foot
PVD is common among patients with diabetes. IC is twice as common among diabetic patients as among nondiabetic patients. An increase in HgbA1C by 1% can result in more than a 25% risk of PAD. Major amputation rates are five to ten times higher in
diabetics than nondiabetics. Because of these causal relations, the American Diabetes Association recommends ABI screening every 5 years in patients with diabetes. Insulin-dependent diabetic patients may have calcified walls of the medium and small arteries that can falsely elevate the segmental pressures of the leg. In this situation, digital pressures of the toes can be accurately measured and a pressure higher than 30 mm Hg is predictive of healing after local amputation and debridement.
diabetics than nondiabetics. Because of these causal relations, the American Diabetes Association recommends ABI screening every 5 years in patients with diabetes. Insulin-dependent diabetic patients may have calcified walls of the medium and small arteries that can falsely elevate the segmental pressures of the leg. In this situation, digital pressures of the toes can be accurately measured and a pressure higher than 30 mm Hg is predictive of healing after local amputation and debridement.
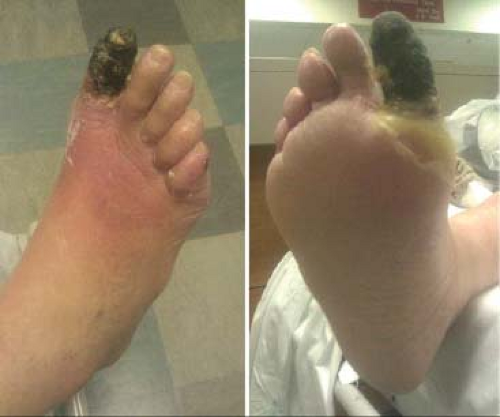
On presentation, a careful physical examination is important to plan for appropriate treatment (Fig. 39.4). The overlying cellulitis is assessed, and any possible underlying abscess is examined by palpation for crepitus or detection of drainage of purulent fluid. Cellulitis should not be confused with dependent rubor caused by severe ischemia in patients with PAD. The presence of an abscess requires immediate drainage prior to revascularization.
In infections with only cellulitis and no underlying soft tissue involvement, patients are treated with IV antibiotic therapy. If the cellulitis does not resolve in several days, there may not be adequate antibiotic coverage and the presence of deep tissue infection is considered. The choice of the antibiotics used and the foot need to be reevaluated; reimaging the foot may be necessary.
The cause of persistent cellulitis and nonhealing infection is usually underlying deep infection or osteomyelitis. Other patients may present with gangrene, open joint or exposed bone, or abscess. In these patients, surgical debridement is required in addition to antibiotic therapy. Small open wounds can be treated with simple debridement, but often there is deep tissue involvement that is not visible on the surface. To remove all nonviable tissue and wide drainage, amputation may be required. If there is extensive infection of the foot with gas, calf pain, or systemic sepsis, the patient may require amputation as an initial therapy. After surgical debridement, patients are treated with aggressive wound care using dressing changes and continued, broad-spectrum antibiotic therapy until intraoperative culture sensitivities are finalized and allow for the use of targeted antimicrobials. Wounds are evaluated closely for persistent infection that may require additional surgical intervention. In patients with adequate arterial circulation, the wound can be closed secondarily after resolution of the infection.
All patients with evidence of concomitant arterial occlusive disease are considered for lower extremity revascularization with open bypass surgery or endovascular stenting or angioplasty to optimize wound healing and limb salvage.
TASC
There are fewer areas in medicine today in which treatment algorithms are changing more rapidly than in arterial occlusive disease. Currently, the decision for revascularization is based on the risks for the surgical intervention balanced against the expected
benefits, including the durability of the treatment and options for further intervention if there is recurrence of symptoms. Rapid advances in endovascular techniques and devices have made the therapeutic decision making process increasingly complex; opinions about which therapies should be used first are varied. In an effort to characterize patients and their lesions and provide guidance about open versus endovascular alternatives, the Trans-Atlantic Inter-Society Document on Management of Peripheral Arterial Disease (TASC) was written and published in January 2000. As practice patterns matured, a second TASC II document was released later in the decade, in 2007. These documents provided classifications of aortoiliac and femoropopliteal disease and strategies for their treatment (Tables 39.1 and 39.2). TASC II recommendations state the following:
benefits, including the durability of the treatment and options for further intervention if there is recurrence of symptoms. Rapid advances in endovascular techniques and devices have made the therapeutic decision making process increasingly complex; opinions about which therapies should be used first are varied. In an effort to characterize patients and their lesions and provide guidance about open versus endovascular alternatives, the Trans-Atlantic Inter-Society Document on Management of Peripheral Arterial Disease (TASC) was written and published in January 2000. As practice patterns matured, a second TASC II document was released later in the decade, in 2007. These documents provided classifications of aortoiliac and femoropopliteal disease and strategies for their treatment (Tables 39.1 and 39.2). TASC II recommendations state the following:
Table 39.1 TASC II Classification for Aortoiliac PAD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
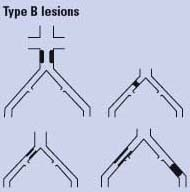
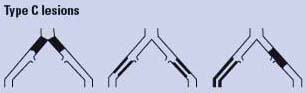
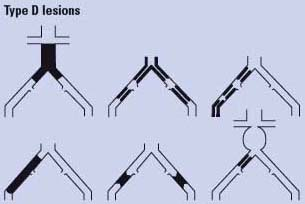
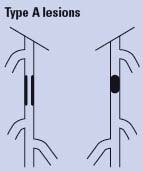
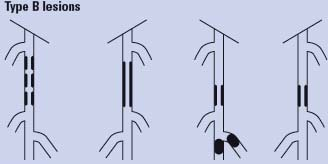
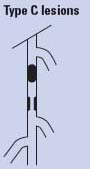
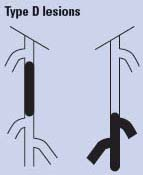
Endovascular therapy is the treatment of choice for type A lesions and surgery is the treatment of choice for type D lesions. Endovascular treatment is the preferred treatment for type B lesions and surgery is the preferred treatment for good-risk patients with type C lesions. The patient’s comorbidities, fully informed patient preference, and the local operator’s long-term success rates must be considered when making treatment recommendations for type B and type C lesions.
Arguably, as long as endovascular intervention does not negatively affect a patient’s option to have an open surgery in the event of restenosis or reocclusion, endovascular
intervention can be attempted for even complex lesions. We routinely treat TASC A through D lesions with endovascular techniques as our initial management strategy.
intervention can be attempted for even complex lesions. We routinely treat TASC A through D lesions with endovascular techniques as our initial management strategy.
Table 39.2 TASC II Classification for Femoropopliteal PAD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
Optimal medical management remains the foundation of treatment in all patients with vascular disease (Table 39.3). Lifestyle modifications with risk factor reduction include exercise, healthy diet, exercise, and smoking cessation. Optimal medical therapy involves strict blood glucose and hypertension control with antiplatelet and statin therapies. Patients with IC are often adequately treated with lifestyle medication and optimal medical therapy since these treatments lead to slow progression of disease with low risk of amputation or limb loss. However, in patients with lifestyle-limiting claudication, rest
pain, or tissue loss there may be compelling reasons to intervene. For the claudicants it is to enhance quality of life while allowing for more effective exercise activity; for those with critical limb ischemia intervention is imperative given their higher risk of limb loss or amputation. Open surgical lower extremity bypass in infrainguinal arterial disease has resulted in excellent outcomes for many years. But, there are qualifiers. Although the cumulative life-table patency rates of infrapopliteal vessels is 47% at 5 years, many patients do not have acceptable venous conduit and the use of suboptimal conduit results in lower patency rates. Five-year patency rates below 25% are sometimes seen in patients with prosthetic graft material. In addition, the 30-day mortality for all patients undergoing infrainguinal arterial revascularization for threatened limbs can be as high as 6%, with major complication rates ranging from 20% to 50%.
pain, or tissue loss there may be compelling reasons to intervene. For the claudicants it is to enhance quality of life while allowing for more effective exercise activity; for those with critical limb ischemia intervention is imperative given their higher risk of limb loss or amputation. Open surgical lower extremity bypass in infrainguinal arterial disease has resulted in excellent outcomes for many years. But, there are qualifiers. Although the cumulative life-table patency rates of infrapopliteal vessels is 47% at 5 years, many patients do not have acceptable venous conduit and the use of suboptimal conduit results in lower patency rates. Five-year patency rates below 25% are sometimes seen in patients with prosthetic graft material. In addition, the 30-day mortality for all patients undergoing infrainguinal arterial revascularization for threatened limbs can be as high as 6%, with major complication rates ranging from 20% to 50%.
Table 39.3 Optimal Medical Management of PAD | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
While long-term results may be less durable, endovascular intervention for patients with lower extremity arterial disease continue to grow in popularity. Recent improvements in guidewire, catheter, stent, atherectomy technology, and catheter-based skills and device alternatives, have transformed the treatment of lower extremity peripheral arterial disease from a primarily open surgical bypass modality to one where endovascular treatment is typically attempted first and may be all that is required. Indeed, many interventionists who treat critical limb ischemia consider endovascular therapy as the first line of treatment in all levels, including infrapopliteal lesions involving tibial vessels. Although it has generally been reported that endovascular patency rates have been lower, limb salvage rates appear similar compared to open surgical bypass patients. In a meta-analysis of open surgical bypass of infrapopliteal arteries, the 1- and 3-year limb salvage rates were 89% and 82%, respectively. This is similar to the limb salvage rates achieved by endovascular treatments of infrapopliteal lesions, 86% and 82%. This is likely that patients with critical limb ischemia require the revascularization be patent long enough for wound healing.
When performing endovascular interventions, certain principles should be followed. First, at least one good distal outflow artery is preserved, so that open surgical bypass to the outflow artery can be performed if endovascular procedure failed. Second, the initially patent arterial segment should not be damaged during intervention. This can avoid unnecessary more distal bypass than would have been required. Third, important collateral circulation should be preserved. This allows patients to return to baseline clinical status if the treated arterial segments reocclude. When these principles are followed, recurrent arterial disease usually does not jeopardize future bypass options.
Key advantages of endovascular procedures include the ability to perform the procedure with the patients under sedation and local anesthesia without the need for general or spinal anesthesia. Perioperative complication rates are significantly reduced compared with open surgical bypasses. Endovascular treatment modalities can be performed without the need for acceptable conduits. The use of percutaneous access avoids the needs for lower extremity incisions, thereby eliminating wound complications. After percutaneous access, most patients are able to ambulate and return to preoperative functional status earlier. Endovascular interventions do not compromise future open bypass surgical options if there is restenosis or recurrence of symptoms.
Vascular Access
The most common complications from endovascular intervention occur at the vascular access site. These include groin hematoma, arterial occlusion, pseudoaneurysm, and arteriovenous fistula. Therefore, meticulous technique used for vascular access is of upmost importance. At our institution, we have adopted three confirmatory steps to ensure safe percutaneous access. (1) Real-time ultrasound guidance is used in all patients to access the contralateral common femoral artery with a 4-Fr micropuncture kit (Fig. 39.5). The ultrasound is used to image the groin vasculature from the distal external iliac artery to the common femoral bifurcation. The common femoral artery is
assessed for any wall calcification or plaque (Fig 39.6A



assessed for any wall calcification or plaque (Fig 39.6A
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree