Endovascular Treatment of Aortoiliac Disease
Sean P. Lyden
David M. Hardy
Peripheral vascular disease (PVD) affects 5% of the population above age 50. The types of symptoms experienced by individuals with PVD are influenced by the location and degree of blockages. Three different patterns of disease presentation have been described based on anatomic location.
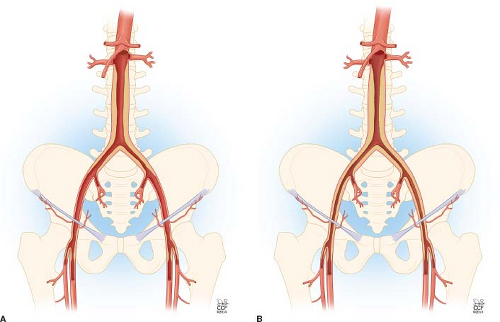
Type I atherosclerosis involves the infrarenal aorta and common iliac arteries only. This pattern of atherosclerosis is present in about 5% to 10% of patients with peripheral arterial disease (PAD) and occurs more commonly in women. The vessels distal to the common iliac arteries usually are generally normal or only minimally diseased (Fig. 37.1A).
Type II atherosclerosis involves the infrarenal aorta, common and external iliac arteries, and may extend into the common femoral arteries (Fig. 37.1B). This pattern is observed in 35% of patients with PAD.
Type III atherosclerosis is the most severe and most common. This pattern of atherosclerosis involves the infrarenal aorta, iliac, femoral, popliteal, and tibial arteries. Endovascular treatment of aortoiliac occlusive disease can be utilized for all these types of disease patterns.
Isolated aortoiliac occlusive disease causes buttock, hip, and proximal thigh claudication. When concomitant distal vessel disease is present the symptoms may include calf and foot claudication or signs of critical limb ischemia (rest pain, ulceration, and gangrene). Indications for treatment of aortoiliac occlusive disease include the elimination of lifestyle limiting claudication, ischemic rest pain, ulceration, and gangrene.
Lifestyle-limiting claudication is typically first managed with a trial of a graded exercise regimen. For isolated aortoiliac disease, endovascular treatment is commonly used without a prior trial of a graded exercise regimen due to the low risk of complications and the good long-term results.
The Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) document has suggested management criteria for aortoiliac occlusive disease based on lesion location and length. I have not found these criteria useful in determining who will benefit from endovascular treatment as this does not take into account individual
skill sets or expertise. TASC II D lesions can be treated with endovascular means with good outcomes in skilled hands.
skill sets or expertise. TASC II D lesions can be treated with endovascular means with good outcomes in skilled hands.
Combination or hybrid therapy using surgical endarterectomy of femoral occlusive disease combined with aortoiliac stenting is a key component to extending minimally invasive therapy to patients with more extensive disease.
Concomitant aneurysmal and aortoiliac occlusive disease traditionally has been treated with open repair. However, off-label use of aortic stent grafts and covered stents has extended the endovascular treatment to this particular anatomy in select cases. Endovascular recanalization of the native aortoiliac segment with vessel patch angioplasty has also been used as successful strategy to treat infected end-to-side aortobifemoral grafts.
There are relatively few contraindications to endovascular treatment of aortoiliac disease. Prior surgical intervention with ligation of native circulation and end-to-end anastomoses is the most common issue in my practice. Coral reef plaque is a relative contraindication, as I believe this is still best treated with open surgery in good risk individuals. Angioplasty of coral reef plaque is associated with issues of both inadequate vessel expansion and vessel rupture.
Identification of aortoiliac disease begins with physical examination.
Diminished or absent femoral pulses should suggest the existence of aortoiliac disease.
Segmental limb pressures with plethysmography will show a drop in the high thigh pressure compared to the brachial artery. The high thigh pressure is typically equal to or 10% greater than the brachial pressure. Pulse volume recordings in the high thigh will be dampened.
Ultrasonography can be very helpful to identify the location, length, and degree of stenoses and occlusions in the aorta, iliac, and femoral arteries. Measurement of native artery diameter helps in defining a goal diameter to achieve with stenting as well as identifying aneurysmal changes. Obesity, bowel gas, and dense calcification can limit the penetration of ultrasound. Evaluation of the common femoral artery will aid in assessing areas to access and identify severe disease needing concomitant femoral endarterectomy.
Computed tomographic angiography (CTA) with runoff has become my imaging modality of choice. CTA should be done from the diaphragm to the toes. With newer generation multidetector CT technology, the machine can often image faster than the passage of contrast, necessitating obtaining delayed imaging to better identify the patent infrageniculate runoff vessels. The native vessel diameter, location and length of blockages, degree of calcification, and prior surgical anatomy can all be identified with CTA. Dual energy scanners may eventually overcome the difficulty of accurately determining contrast from calcification in distal vessels.
Magnetic resonance imaging with angiography can be used to evaluate the extent of the disease process. Magnetic resonance imaging does have a limitation of signal dropout suggesting vessel occlusion in areas with low flow or prior stents.
Combining physical exam and the anatomic locations of the blockages, a treatment strategy can be developed.
When only aortic, and iliac stenoses exist and the femoral arteries are spared, use of either unilateral or bilateral groin percutaneous access should suffice. When disease is located at the aortic bifurcation bilateral access improves imaging and placement accuracy. Once occlusions are present, use of antegrade and retrograde access increases the chance of successful crossing. Antegrade access can be from the contralateral femoral artery but usually the direct force vector into the ostia of the vessel is better created using brachial or less commonly radial access.
Positioning and Prep
Although these procedures can be done in a standard operating room with a mobile C-arm, use of a fixed imaging system provides better image quality. Fixed imaging systems also allow for higher magnification and image resolution and does not have issues with overheating that may occur with portable units. The built-in lead shielding to the table and room also limits radiation scatter from the patient and the dosage incurred by the treatment team. A floating point table can allow movement over a large area quickly when achieving access speeding up the procedure.
When only percutaneous intervention is planned, the area around the left brachial artery and umbilicus to knees are prepped. When concomitant open iliofemoral endarterectomy is planned, the patient is prepped from the chest to the knees to allow for retroperitoneal or aortic exposure if needed as a surgical bailout. Patients with impaired renal function, defined as glomerular filtration rate (GFR) less than 60, should be hydrated with isotonic fluids for 3 hours prior to iodinated contrast exposure to minimize nephrotoxicity. Several studies have suggested the usage of N-acetyl cysteine given 600 mg oral twice daily on the day prior and the day of contrast exposure will reduce the risk of contrast-induced nephropathy but this has not been supported by meta-analysis. Patients with iodinated contrast dye allergies are premedicated with oral prednisone 50 mg given 12 hours and 1 hour prior to contrast administration. Benadryl 25-mg tablet is also given orally 1 hour prior to contrast administration.
Access Technique
Ultrasound guidance using micropuncture techniques are highly recommended. For patients with patent but narrowed iliac vessels, access on the least narrowed side is usually the best place to begin. The ultrasound can identify the femoral circumflex vessels as well as the origins of the superficial femoral and profunda femoral vessels. Access on the top of the midpoint of the common femoral artery away from significant occlusive lesions and dense calcification is important to minimize risk of vessel damage at the completion of the procedure. When concern exists of the accuracy of the access, injection of contrast through the micropuncture 3-Fr transitional dilator can identify any potential issues prior to upsizing to a larger diameter sheath. Once adequate access is obtained, a hydrophilic wire is directed through the transitional dilator up into the thoracic aorta with use of a torque device.
If preoperative imaging has confirmed the need for aortic or iliac intervention, I will start with placing a 25-cm long 6-Fr diameter marker tip sheath. If no preoperative imaging exists, then placing a short 5-Fr sheath will be used to help minimize the access diameter until decision to intervene is made. Aortic and pelvic and bilateral lower extremity imaging should be obtained. If lower extremity runoff has been imaged by CTA, I will not repeat it. I prefer to use a Verrill contralateral flush catheter (Cook Inc., Bloomington, IN) since this is a flush catheter but the reverse curve will allow direction of a wire to the contralateral iliac in order to obtain up and over access, without using another catheter. For aortic imaging, I will use a power injector with the rate of rise set at 0.1 second the pressure limit set at 900 psi and inject 15 cc/s for a total of 25 cc. For pelvic imaging I will use same pressure and rate of rise and use 10 cc/s for 15 cc total. In the setting of vessel occlusions larger amounts of contrast with longer injections can be helpful to identify distal patent vessels as they fill through collaterals.
The number of access sites is up to the interventionalist, but I will generally use bilateral 6-Fr 25-cm long marker tip femoral access sheaths when ostial common iliac arteries disease exists. The dilator can be used to advance the sheath into the distal aorta effectively predilating critical stenosis, allowing for delivery of stents. The marker tips are a critical component to allow visualization of the tip of the sheath to avoid deploying stents within the sheath. The long sheaths allow injection into the distal aorta, reducing the amount of contrast and catheter exchanges needed.
Brachial access is used for aortic occlusions and when iliac occlusions are not able to be easily crossed or reentry has not occurred at the ostia of the common iliac artery.
Brachial access from the left arm is preferred over the right arm to minimize the amount of arch crossed which should lower stroke risk.
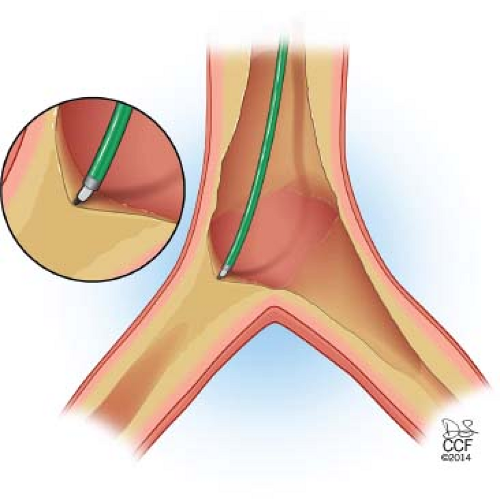
Similar to the femoral access, ultrasound-guided access is obtained over the anterior surface of the brachial artery just at the level of the antecubital fossa. A stiff hydrophilic wire is negotiated through the micropuncture transitional dilator into the arch of the aorta. A pigtail catheter or cobra 2 catheter can be used to direct the wire into the descending aorta and to the level of the occlusion in the aorta or iliac artery. For aortic chronic total occlusions (CTO), imaging can be done through this approach first, especially if thrombolysis is planned. Once imaging is obtained, a long 6-Fr 90-cm sheath is advanced over the wire to the point of the occlusion. A 90-cm long sheath will reach to the aortic bifurcation in just about any individual, improving support and pushability to cross occlusions. A 100-cm long angled catheter is advanced over the stiff hydrophilic wire and the sheath, catheter, and wire tip are stacked up at the point of occlusion (Fig. 37.2).
Stacking the sheath, catheter, and wire close together allows for more pushability and less buckling of the wire allowing entry into the cap of the occlusion and directional control into the iliac artery.
Lesion Crossing
I approach aortic and iliac occlusions differently. For aortic occlusions an antegrade approach from brachial access is preferred.
For short distal aortic occlusions directed stenting is the approach most commonly taken.
For long aortic occlusions or occlusions where the wire traverses easily suggesting thrombus, I will use thrombolysis to unmask the causative lesions allowing for less extensive stenting if possible.
When flush aortic occlusions with the level of the renal arteries, creating of a flow channel with 6-mm balloon angioplasty will decrease the risk of embolism to the renal arteries. I will typically use pharmacologic thrombolysis with a dual level infusion through both an infusion catheter and wire or place ultrasound-enhanced thrombolysis with an EkoSonic mach4E catheter (EKOS Corp, Bothell, WA). I will run concomitant heparin through the brachial access sheath at 500 u/h to avoid clot forming around the access site. I will only place the infusion system down one iliac artery and allow for overnight thrombolysis. When imaging shows improvement and creation of a flow channel, I will redirect the catheter into the opposite iliac artery and continue with thrombolysis for another 24 hours. I will check fibrinogen levels daily and when the fibrinogen falls below 100 mg/dL, I use mechanical thrombectomy devices to debulk the remaining thrombus. When the causative lesions are unmasked stenting will proceed as outlined below.
For common iliac occlusions that are not flush with the aorta, I will first attempt to cross from a retrograde femoral access.
The difficulty in iliac CTO treatment lies in true lumen reentry.
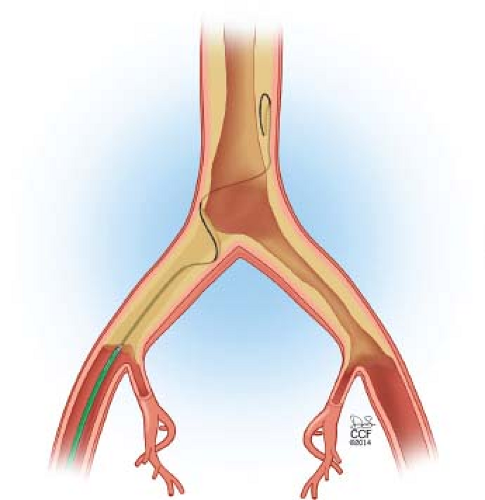
Aortic plaque commonly extends into the common iliac arteries. During retrograde recanalization of iliac CTOs, the wire can enter a deeply subintimal space or track outside of dense calcification, in these instances the wire usually will not be able to penetrate back into the true lumen of the aorta. The wire will continue to pass in this space up into the aorta above the iliac origin (Fig. 37.3). When recannalizing iliac CTO from a retrograde approach, reentry in the distal aorta above the iliac origin may not be recognized, leading to inadequate treatment or requirement to raise the aortic bifurcation. While some authors have suggested the use of reentry catheters as the ideal method to achieve reentry, I have found antegrade recanalization from a brachial approach quicker and more cost effective in these situations.
When the common iliac artery occlusion is flush with the ostia, I will always begin from a brachial artery access.
Bringing a sheath to the level of the occlusion is critical in providing longitudinal support for the catheter to allow for penetration of the cap.
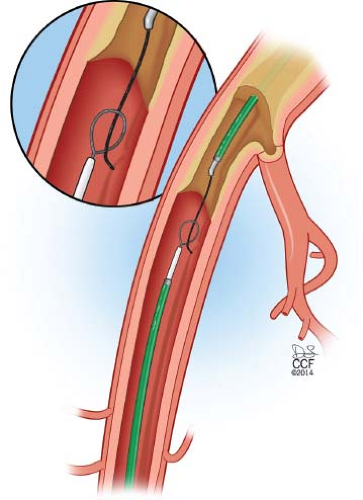
If distal reentry is difficult, retrograde access into the subintimal plane is recommended. Connection of the subintimal planes will allow the wire to be snared from the femoral sheath allowing for through-and-through access (Fig. 37.4). The greatest advantage of this approach is forcing lesion entry with a wire at the ostia of the common iliac artery.
When both common and external iliac arteries are occluded, I will use brachial and bilateral distal femoral access. This will ensure that entry points are flush with the occlusions both proximally and distally. Most commonly the femoral access is used to get up the proximal common iliac artery and the brachial approach is then used to enter into the common iliac and the wires are connected in the subintimal space. In snaring the brachial wire, the true lumen to true lumen access is confirmed. Once this is done I will push a catheter over the through-and-through wire from the femoral approach up into the aorta and confirm crossing by angiography after removal of the wire. A stiff wire can now be placed from the femoral access into the thoracic aorta to allow for treatment. When bilateral occlusions occur this process is repeated on the opposite side.
Isolated external iliac artery occlusions are generally approached from a contralateral femoral access. When reentry into the true lumen is not possible or occurring in the mid or distal common femoral artery, retrograde ipsilateral femoral access can be added.
Iliofemoral Endarterectomy
In patients with significant common femoral and/or distal external iliac occlusive disease, I will start the case with external iliac and femoral endarterectomy and profundaplasty. If the external iliac artery is occluded, the common femoral artery can still be identified in almost all patients by palpating for the diseased artery by rolling fingertips over the groin just distal to the inguinal ligament. A longitudinal skin incision is performed as this allows easy extension down the leg when extensive disease into the profunda femoral artery branches exists. I will first incise the skin, subcutaneous tissue
and ligate any potential lymphatic vessels between silk ligatures to reduce the risk of postoperative lymphatic leak. The femoral sheath is incised in a longitudinal fashion. The anterior surface of the common femoral artery will not have any branches and is a safe plane to dissect in. Exposure is carried cephalad above the medial and lateral femoral circumflex vessel on to the external iliac artery. The vein crossing over the external iliac artery just under the inguinal ligament is identified and divided. This will facilitate exposure of the external iliac artery and blunt finger dissection can be used to free up the anterior surface of the external iliac artery. I will place an Omni-Tract Wishbone retractor (Omni-Tract Surgical, St. Paul, MN) and use a short renal artery blade to retract the inguinal ligament cephalad (Fig. 37.5). This maneuver will allow exposure of the distal 10 cm of the external iliac artery. The dissection of the external iliac artery is done circumferentially to allow safe clamp placement.
and ligate any potential lymphatic vessels between silk ligatures to reduce the risk of postoperative lymphatic leak. The femoral sheath is incised in a longitudinal fashion. The anterior surface of the common femoral artery will not have any branches and is a safe plane to dissect in. Exposure is carried cephalad above the medial and lateral femoral circumflex vessel on to the external iliac artery. The vein crossing over the external iliac artery just under the inguinal ligament is identified and divided. This will facilitate exposure of the external iliac artery and blunt finger dissection can be used to free up the anterior surface of the external iliac artery. I will place an Omni-Tract Wishbone retractor (Omni-Tract Surgical, St. Paul, MN) and use a short renal artery blade to retract the inguinal ligament cephalad (Fig. 37.5). This maneuver will allow exposure of the distal 10 cm of the external iliac artery. The dissection of the external iliac artery is done circumferentially to allow safe clamp placement.
 Get Clinical Tree app for offline access 
|