Fig. 10.1
Anaconda® stent
Endoprótese Aorfix®
AORFIXTM has the most advanced stent technology on the market [9]. It is manufactured by Lombard Medical and marketed in Europe and other countries [1].
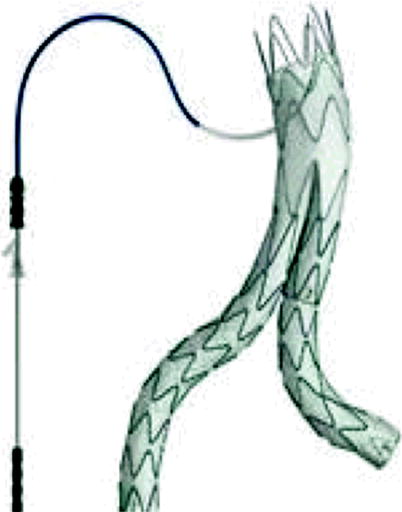
Because of its shape and high flexibility, it fits the anatomy of the patients perfectly [12], optimizing operation performance, also in the postoperative period. Its “fish mouth” design allows optimal positioning in the proximal neck, with the lower portions lateralized, allowing flow to the renal arteries while keeping the highest parts oriented to the anterior and posterior portions of the aorta, ensuring suprarenal fixation. It is made of nitinol, which has a circular framework covered with polyester fabric, ensuring perfect adaptation of the graft, even after surgery. Radiopaque markers ensure correct positioning during the procedure, and fixing hooks ensure safer fixation [9].
For aneurysms with significant angulation of the proximal neck (60–90°) and tortuous iliac arteries, the use of this stent has proved to be extremely effective, resulting in low rates of type I endoleaks and iliac branch occlusion. Angles greater than 60° are a contraindication for the implantation of other available devices. Therefore, the development of Aorfix ® has enabled the extension of endovascular treatment to aneurysms with complex anatomies [13].
This device is available with proximal diameters of 24–31 mm, with a range of 1 mm. The length of the main body, provided by the manufacturer, is the aortal portion and has a size range of 86, 96, 111, 126, and 142 mm. The diameter of the distal ipsilateral branch can vary from 10 to 20 mm, increasing each 2 mm, and has lengths of 63, 80, and 97 mm. The contralateral branch has a proximal diameter of 12 mm, and the distal one, with the same variety of diameters as the distal ipsilateral branch, has lengths of 56, 64, 73, 81, 90, 98, and 105 mm [10] (Fig. 10.2).
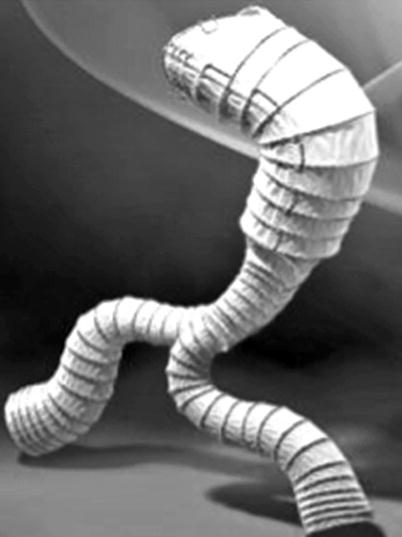

Fig. 10.2
Endoprótese Aorfix®
Endoprótese Apolo®
This is produced by Nano Endoluminal, a Florianópolis-Santa Catarina company. It was the first Brazilian stent and has been produced since 1998.
It is available in monoiliac and bifurcate formats, both with a woven metal structure formed by a single nitinol wire and coated with high-strength PTFE [14].
It is a modular, auto-expandable endoprosthesis designed for vascular reconstruction of infrarenal abdominal aneurysms. It presents a free proximal stent and barbs that ensure excellent fixation and reduce the possibility of device migration. It features gold radiopaque markers, which help in the visualization and accurate positioning of the stent [15].
The main body of the bifurcated Apollo® has diameters of 25, 28, 31, and 34 mm, and the length of the covered portion ranges from 130 to 180 mm. The monoiliac Apollo® has the same diameters as the bifurcated one, but the length ranges from 130 to 175 mm. Both have distal diameters of 12, 14, 16, and 18 mm. The straight distal iliac branches have diameters from 12 to 18 mm and lengths ranging from 60 to 115 mm. The conic iliac branches have proximal diameters of 12, 14, 16, and 18 mm and the distal ones 16, 18, 25, and 28 mm and lengths ranging from 80 to 130 mm. Proximal extensions are also offered with a length of 45 mm. Occluders have diameters of 12, 14, 16, 18, 25, and 28 mm and extension of 25 mm. Additionally, stents can be customized, with the size and design set by the physician. Thus, one can include fenestrated and branched stents [10].
Endoprótese Braile®
The new generation of stents manufactured by Braile Biomedica, a company from Sao Jose do Rio Preto-Sao Paulo, is composed of a metal frame coated with polyester. It has high flexibility and radial strength, and its release is caused by mobilization of the simple sheath. It also has proximal and distal radiopaque markers to facilitate stent placement and location [3–16]. It is indicated for the correction of abdominal aortic aneurysms (AAA) and iliac arteries.
Its main body has a proximal diameter from 24 to 36 mm and a distal one of 14 mm, with lengths of 80, 130, 155, and 170 mm. The free flow length ranges from 20 to 25 mm. It has a low profile that varies from 18 to 20 F [17].
Endoprótese Ella®
Ella is manufactured by CS Ella in the Czech Republic. Treatment of AAA with this stent is effective and safe. It has been used in Brazil since 1998 with good results.
It has a resistant coating of polyester mesh attached to the stent by ultra-resistant polypropylene sutures and has two rows of barbs for stabilization and fixation to prevent migration of the stent, branches, and modular extensions that also have barbs for better fixation. It has high radial force on a continuous metal skeleton, radiopaque markers indicating the ends of the stent and the beginning of the coated portion, and a smooth surface at any angle, without risk of pressure at any site (maximum angle of 60°) [1–10].
It is indicated for less invasive alternative treatment of aortic aneurysms (AA), anastomotic pseudoaneurysms, or aneurysms without signs of infection. It can also be used to occlude the false lumen in case of dissection of the aortal portion occurring in the descending thoracic aorta and abdominal aorta or even to occlude the aorto-cava fistulas [19].
The main body has a bifurcated proximal diameter ranging from 14 to 38 mm and a distal one from 6 to 20 mm with lengths from 110 to 220 mm. These measures also apply to the monoiliac model. The iliac branch has diameters ranging from 6 to 20 mm and lengths from 22 to 120 mm. The iliac occluder has diameters from 12 to 30 mm and lengths from 35 to 60 mm (Fig. 10.4).
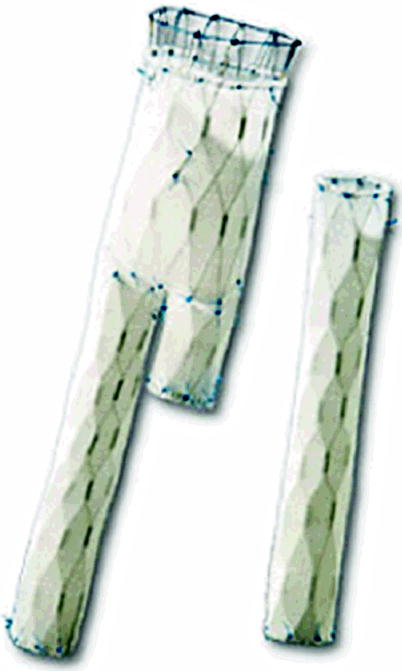

Fig. 10.4
Endoprótese Ella®
Endoprótese Endofit®
This is produced by LeMaitre Vacular, Inc. (Burlington, MA, USA), and consists of a unique, safe, and effective aortomonoiliac device [20]. Its nitinol structure has a conical shape and layers of PTFE, without sutures. Its attachment is positioned at the suprarenal level with a free 28 mm stent, and radiopaque platinum markers indicate the initial site of the device [1–12].
Its delivery system is simple and can be positioned above the place indicated as it does not have fixation barbs. One should retract the introducer and then adjust its position [22].
The stent has proximal diameters of 26, 28, 30, 32, 34, or 36 mm, a distal one of 16 mm, and a length of 160 mm. There is also an 180-mm-long stent with a proximal diameter of 34 and 36 mm [22].
Distal straight tubular iliac extension has a diameter of 16 or 18 mm with a length of 80 or 100 mm. The proximal aortic extension stent is a free stent, is 50 mm long, and has the same diameter as the main body. Also the proximal extension is 38 mm long, which is compatible with the 24 F introducer. The iliac occluder has diameters of 18, 20, 23, 24, and 26 mm and is 35 mm long, being compatible with the 20 F introducer for 26 mm diameter and 18 F for other diameters [10] (Fig. 10.5).
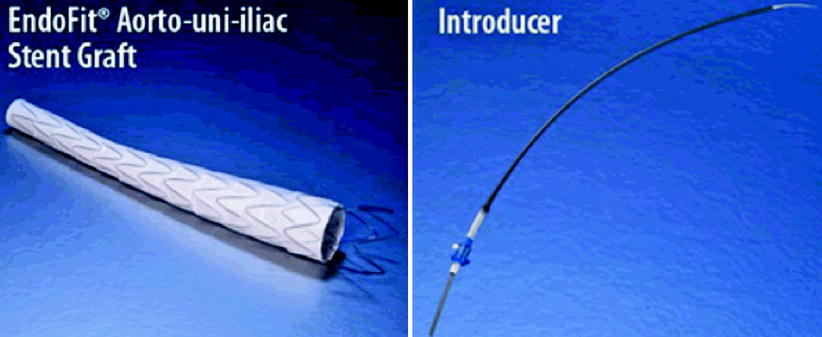

Fig. 10.5
Endoprótese Endofit®
Endoprótese Endurant®
This device is produced by Medtronic Vascular (Santa Rosa, CA). It is a last generation device designed to expand the applicability of endovascular repair of the aorta (EVAR) [24]. It was approved for use by the US Food and Drug Administration (FDA) on 16 December 2010. Its use is authorized for necks ≥ 10 mm and up to 60° angulation [24].
The endoprosthesis is made of a metallic material (nitinol) coated with polyester and fixed by polyethylene sutures. It is auto-expandable, hydrophilic, and has a low profile. It has radiopaque markers that look like the letter “e” in the proximal end, at the level of bifurcation, and in the distal end, which allow adequate positioning. The upper end has a free-flow portion composed of a single filament of nitinol. Fixing is achieved by means of a free-flow portion, which has barbs, and by the radial strength of the system [10–23].
The Endurant®device is safe and effective, with an excellent rate of successful implementation and very low leakage and reintervention rates [22].
The diameter of the main body is from 23 to 25 mm (introducer 18 F) and from 28 to 36 mm (introducer 20 F), and the length ranges from 120 to 170 mm. The iliac branches have distal diameters from 10 to 28 mm and lengths ranging from 80 to 120 mm. The iliac branches and extensions are also provided for by the “bell bottom” shape and by the distal diameter being larger than the proximal one in order to treat dilated iliac arteries. Aortouniiliac stents are available as well (Fig. 10.6).


Fig. 10.6
Endoprótese Endurant®
Endoprótese E-Evita®
E-vita® is produced by Jotec (Hechingen, Germany). It is a relatively new infra-renal stent, having been approved for clinical use in Europe since 2008. It is a self-expandable modular device, and one of its features is supra-renal fixation [2, 25]. It has low porosity, with minimal blood loss [2] and high flexibility, being suited to the anatomy of aneurysms with neck angulation or tortuous and dilated iliac arteries [2].
It contains individual nitinol stents, coated with polyester mesh in tubular or conical shapes. The first ring is not covered and is part of the local fixation [1].
Its delivery system is marked by a “squeeze-to-release” approach, which allows the stent to be released in a controlled and constant manner by means of pressing a trigger [2].
The catheter tip is long and flexible, allowing percutaneous introduction and easy tracking through vessels. The marking ring on the catheter sheath provides information about the degree of stent release [2].
The main body is available in two different lengths (150 and 170 mm) with trunk lengths of 50 and 70 mm, which can be combined in any manner, allowing optimization of the treatment. Its proximal diameter varies from 24 to 34 mm, and the distal one is 14 mm for the short leg and from 12 to 22 mm for the long leg. The contralateral branch presents a proximal diameter of 16 mm and distal one from 12 to 24 mm [26, 27, 28]. The length varies from 50 to 105 mm [12] (Fig. 10.7).
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree