Endovascular Repair of Thoracoabdominal Aortic Aneurysms
W. Anthony Lee
Introduction
A thoracoabdominal aortic aneurysm (TAAA) is a pathologic dilation of the aorta involving both its thoracic and abdominal segments, and as such may involve the origins of the four main visceral arteries (celiac, superior mesenteric, and/or right and left renal arteries). While TAAA is most commonly associated with a degenerative, atherosclerotic etiology, other etiologies may include chronic dissections, postsurgical complications, primary or secondary infections, and connective tissue disorders. If left untreated, all TAAA may be complicated by progressive dilation, pain, and rupture, leading to patient death.
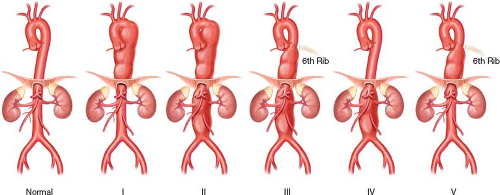
TAAA have been classified according to the proximal and distal extents of the aneurysmal segment (Fig. 33.1). Briefly, Type 1 starts above the 6th intercostal space and extends to the celiac artery; Type 2 starts above the 6th intercostal space and extends below the renal arteries; Type 3 starts at or below the 6th intercostal space and extends below the renal arteries; Type 4 starts above the celiac artery and extends below the renal arteries; and finally, Type 5 starts at or below the 6th intercostal space and extends to the celiac artery. The classification scheme has prognostic significance and correlates with the risks of spinal cord ischemia (SCI), death, and other major complications.
Current standard of care for repair of TAAA is open surgical repair (see Chapters 8 and 16). However, the morbidity and mortality associated with this procedure are substantial and exceed those associated with almost every elective vascular surgical procedure. A second potential treatment option, which became transiently popular in the last decade, is a “hybrid” repair, which combines open and endovascular techniques to avoid aortic cross-clamping, reperfusion, and reduce visceral ischemia (Chapter 46). The fundamental concept of this approach is to create a landing zone proximal and/or distal to the aneurysmal segment of the aorta. It involves mesenteric and/or renal revascularization or “aortic debranching” using a complex network of extra-anatomic bypasses that is followed by deployment of a commercially available endograft system. The third approach is a total endovascular repair. This approach involves the use of branched and/or fenestrated endografts or so-called “snorkel” or “chimney” techniques, which involves
placement of visceral stents outside of, but parallel, to a conventional endograft, to maintain visceral branch perfusion.
placement of visceral stents outside of, but parallel, to a conventional endograft, to maintain visceral branch perfusion.
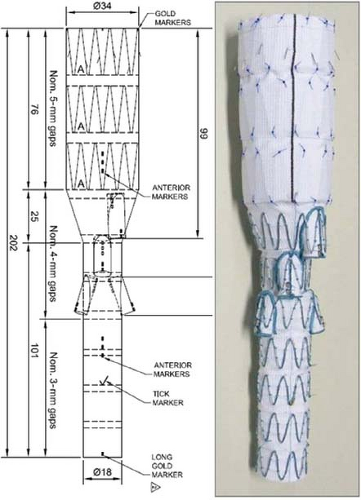
Recently, a standardized, multibranched aortic endograft (Zenith® t-Branch Endovascular Graft, Cook Australia, Brisbane) was developed in an effort to provide an “off-the-shelf” solution to endovascular TAAA management (Fig. 33.2). This device is currently
available in the United States under an FDA (Food and Drug Administration) investigational device exemption (IDE), but commercially available outside the United States. The use of this device in the endovascular repair of a TAAA is the subject of this chapter.
available in the United States under an FDA (Food and Drug Administration) investigational device exemption (IDE), but commercially available outside the United States. The use of this device in the endovascular repair of a TAAA is the subject of this chapter.
Device Description
The Zenith t-Branch device is based on the Zenith TX2 thoracic endograft platform and shares its basic construction such as barbs for proximal active fixation, stainless steel Z-stents attached either as an endo- or exoskeleton to a full-thickness polyester fabric. More specifically, however, the t-Branch device is 202 mm long with a 34-mm proximal diameter which tapers in its mid-segment to 18 mm. It consists of four caudally oriented branches, each serving as the attachment site for a covered stent that connects the branch to the visceral artery (Fig. 33.2). From proximal to distal, the diameter, length, and clock-orientation of the branches correspond to the celiac (8 × 21 mm, 1:00), SMA (8 × 18 mm, 12:00), right renal (6 × 18 mm, 10:00) and left renal (6 × 18 mm, 3:00). Radiopaque markers assist in orienting the t-Branch to the optimal orientation for catheterization of the native visceral arteries and showing the proximal and distal ends of the branches.
The delivery system is leveraged off the TX2 and Zenith Fenestrated endograft systems and enclosed in a kink-resistant, braided 22-Fr (French) (outer diameter, O.D., 8.5 mm) hydrophilic sheath. It employs a series of trigger wires that secure the proximal and distal ends of the endograft to the delivery catheter, and maintain the so-called diameter reducing ties that partially constrain the endograft after it is unsheathed in vivo to allow some degree of rotational correction.
The t-Branch device is completely modular and mates with the TX2 thoracic proximal component proximally, and a “universal” bifurcated endograft (Unibody) that is almost identical to the Zenith Flex AAA device (but without barbs or the bare, suprarenal stents) distally that is delivered in an 18-Fr (O.D. 6.7 mm) delivery system. Standard iliac limbs are used to extend and complete the aortic portion of the t-Branch repair.
Size and symptoms are the principal indications for treatment. Symptoms such as severe, unusual, and unremitting back pain that may be attributed to the aorta represent an unequivocal indication for urgent repair unless there are other mitigating circumstances. In general, 6 to 6.5 cm is considered an acceptable threshold for recommending elective repair in most patients. Natural history studies have shown that the risk of rupture substantially increases when the TAAA reaches 7 cm.
Absolute contraindications are few but there are a number of relative contraindications for a t-Branch repair, which mostly pertain to anatomic issues.
Proximal thoracic landing zone diameter >38 mm
Supraceliac aortic angulation >90 degrees
Paravisceral patent luminal aortic diameter <26 mm
Occluded subclavian arteries
Lateral or upgoing renal artery
Short (<15 mm) main visceral trunk proximal to vital branch (e.g., replaced hepatic artery off SMA, early bifurcation of left renal artery)
One study showed that this off-the-shelf device was potentially applicable in 88% of the patients who were eligible for endovascular repair with a custom-manufactured stent-graft, and in an initial series involving 38 patients all 136 branches were able to be inserted as intended using this off-the-shelf device, without migration, disconnection, or kinking.
Preoperative planning is critical to successful completion of a t-Branch repair. All patients routinely undergo a full cardiac workup to look for clinically significant coronary artery disease for risk stratification and medical optimization. Antihypertensive medications are held for 24 hours and transient hypertension is tolerated. SCI and paraplegia/paresis is one of the most dreaded complications after TAAA repair. A preoperative spinal drain is placed in everyone unless there is an anatomic (e.g., previous spinal surgery or instrumentation, severe scoliosis) or medical contraindication to minimize this risk. To this end, anticoagulants and antiplatelet agents are held for a requisite number of days prior to surgery to avoid the risk of hemorrhagic complications. Although local practices differ widely, there are certain advantages to preoperatively admitting the patient and inserting the spinal drain and other invasive hemodynamic monitoring lines the day before surgery.
All patients should have a thin-cut CT angiogram (CTA) of the chest, abdomen, and pelvis. For those with chronic kidney disease, typical measures are used to reduce the risk of contrast nephropathy and a contrast scan is still performed. There is no substitute for a high-quality CTA, and any risks of a contrast study (acute kidney injury and/or anaphylaxis) must be considered as part of the therapy itself and in the context of the alternative of leaving the TAAA unrepaired.
The CTA dataset is next postprocessed using one of several 3D DICOM imaging tools (e.g., TeraRecon) that can reformat the data into volumetric and multiplanar renderings. A properly performed CTA allows a detailed review of the TAAA anatomy itself, as well as assessment of the axillary and subclavian arteries (diameter and presence of occlusive disease), arch morphology, presence of any atheromatous disease, and iliofemoral arteries for t-Branch delivery. This is necessary to formulate a “plan of attack” for introduction of the myriad of endovascular devices that will be necessary for a successful repair.
Case planning is fairly straightforward and consistent from case-to-case. Briefly:
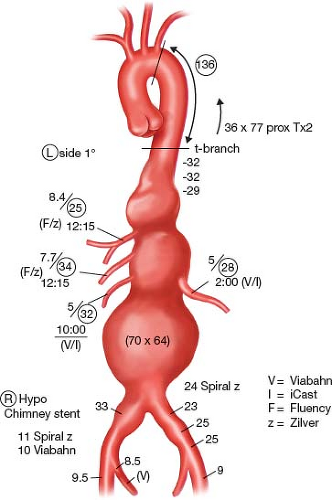
Gross morphologic assessment is made from a volume rendering looking for aortic and visceral vessel tortuosity and angulation. Severe angulations of the supradiaphragmatic aorta are common in TAAA (Fig. 33.3). This can adversely
impact delivery and orientation of the t-Branch device and catheterization of the branches.

Figure 33.3 Type II TAAA. Note the prior EVAR of an infrarenal AAA using the Medtronic AneuRx device (Santa Rosa, CA).
Centerlines are generated through the entire thoracoabdominal aorta, both iliac arteries and each of the visceral vessels. This allows true (orthogonal) diameter measurements.
Outer diameters and lengths of thoracic aortic and iliac landing zones are measured. The proximal landing zone requires some judgment. The objective is to select the most distal segment of the thoracic aorta that will minimize coverage of nonaneurysmal aorta (to reduce the risk of SCI), provide a secure proximal seal and fixation of the t-Branch, and leave at least 10 to 15 mm distance from the distal margins of the branches to their respective visceral vessels. This distance is further adjusted proximally or distally in cases of excessive thoracoabdominal angulation to avoid landing the branch segment of the device near the apex of the bend. The minimum distance between the end of the branch and the target vessel is necessary to facilitate catheterization of the native artery without the need for precise registration, as would be required of a fenestrated device. This singular operational concept forms the cornerstone of an “off-the-shelf” thoracoabdominal device.
Inner (luminal) diameters, lengths of the common trunk, and clock-orientations of all four visceral vessels are measured (Fig. 33.4).
The sizes and types of any thoracic components (not always necessary), iliac limbs, and branch (bridging) stents (covered, uncovered) are selected. Lengths of some of these devices can only be determined intraoperatively, and therefore a wide array of sizes in multiples should be available.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree