Endovascular Repair of Juxtarenal Aneurysms
John C. McCallum
Matthew J. Alef
Marc L. Schermerhorn
Indications
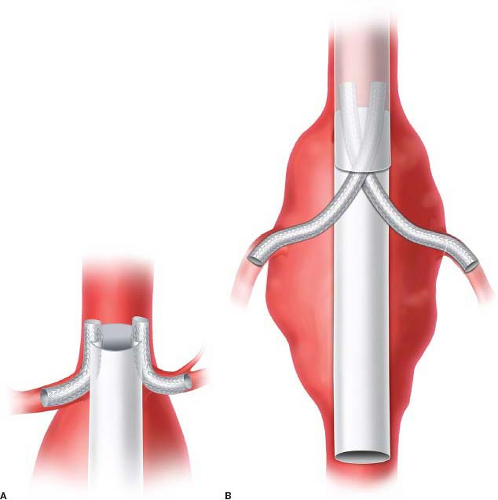
Traditionally, juxtarenal abdominal aortic aneurysms (AAA) are defined by an inadequate length of normal infrarenal aorta for both clamping and sewing below the renal arteries (Fig. 34.1). Thus during open repair the proximal clamp must be placed above at least one renal artery. In the endovascular era, infrarenal aneurysms are identified by presence of greater than 10 mm of normal aorta between aneurysm sac and lowest renal takeoff necessary for standard EVAR. This seal zone requirement is the most common anatomic exclusion criterion precluding patients from receiving EVAR. Juxtarenal aneurysms are effectively defined by the indications for use of currently available endovascular devices as an infrarenal neck of between 4 and 10 mm.
A variety of advanced endografts have been developed to address the problem of foreshortened infrarenal seal zone. Physician-modified grafts have been used with early success, but quality control and liability have limited widespread use and long-term data are lacking. This technique should be used with an investigational device exemption. The current standard for endovascular juxtarenal aortic aneurysm repair employs custom fenestrated endografts with fenestrations (holes) or scallops (U-shaped opening in the top of the graft) that are aligned with the ostia of the renal vessels, and often the SMA, with or without bare or covered stents placed into these vessels. Mimicking aortic branching, custom branched endografts have been developed and are manufactured to patients’ specific anatomy. Branched endografts are being developed into devices for wider use but are not currently commercially available in the United States. Chimney and sandwich techniques employing parallel grafts offer further flexibility for off-label use without device modification, and new techniques are under development. Patients whose aneurysm anatomy exceeds the range of standard EVAR but in whom an endovascular option is preferred may benefit from these techniques.
While this chapter is not intended as a primer on specific devices, at present a single device is FDA-approved for juxtarenal aneurysms in the United States. The
Zenith™ Fenestrated AAA Endovascular Graft (Cook Medical, Bloomington, IN) is approved for patients with a nonaneurysmal infrarenal aortic segment of at least 4 mm in length (Fig. 34.2). This neck length is the anatomic consideration that distinguishes this device, and additional conditions must be met as in other EVAR patients.
Zenith™ Fenestrated AAA Endovascular Graft (Cook Medical, Bloomington, IN) is approved for patients with a nonaneurysmal infrarenal aortic segment of at least 4 mm in length (Fig. 34.2). This neck length is the anatomic consideration that distinguishes this device, and additional conditions must be met as in other EVAR patients.
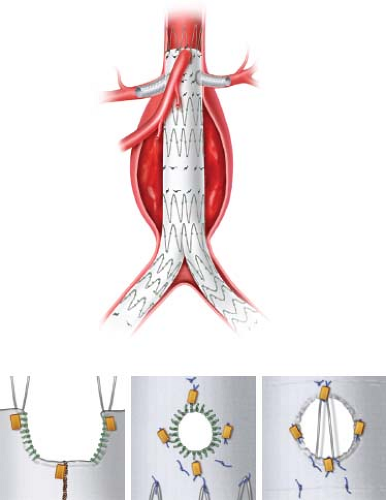 Figure 34.2 A: Cook Zenith Fenestrated Endograft with B: scallop, C: small fenestration, and D: large fenestration. |
Neck outer–outer wall diameter between 19 and 31 mm
Neck angulation less than 45 degrees relative to both short and long axis of the aneurysm
Ipsilateral iliac artery fixation zone at least 30 mm in length and 9 to 21 mm in diameter
Contralateral iliac artery fixation zone at least 30 mm in length and 7 to 21 mm in diameter
An additional fenestrated endograft, the Ventana Fenestrated System (Endologix, Irvine, CA) is in the investigational phase in the United States. Fenestrated grafts offer options both for patients with short necks at the time of first repair, and as a salvage option for patients who have had progression of the aneurysm sac after standard EVAR causing degradation of the infrarenal seal zone.
While fenestrated grafts have been adopted more quickly in the United States for the treatment of juxtarenal aneurysms, an alternative advanced EVAR technique known as a chimney configuration has also been successfully employed and promoted in the United States and abroad. The advantages of the technique include the lack of a need for preoperative customization, the potential for lower cost, and an extension of anatomic criteria.
Not requiring preoperative fabrication or intraoperative device modification, chimney grafts have the potential for use in ruptured AAA and emergency EVAR. As chimney grafts have been studied less rigorously, clear anatomic indications are lacking but initial results suggest a significant range of anatomic fit. A recent review of published studies examining chimney grafts by Tolenaar et al. noted their use in aneurysms with neck lengths between 0 and 11 mm, and neck diameters between 19.8 and 32.2 mm. Similarly to chimney grafts, a sandwich configuration has been used to extend available off-the-shelf stent grafts beyond infrarenal aneurysms. In a sandwich graft, covered stents are positioned in visceral/renal vessels and between two aortic stent grafts (Fig. 34.3).
Not requiring preoperative fabrication or intraoperative device modification, chimney grafts have the potential for use in ruptured AAA and emergency EVAR. As chimney grafts have been studied less rigorously, clear anatomic indications are lacking but initial results suggest a significant range of anatomic fit. A recent review of published studies examining chimney grafts by Tolenaar et al. noted their use in aneurysms with neck lengths between 0 and 11 mm, and neck diameters between 19.8 and 32.2 mm. Similarly to chimney grafts, a sandwich configuration has been used to extend available off-the-shelf stent grafts beyond infrarenal aneurysms. In a sandwich graft, covered stents are positioned in visceral/renal vessels and between two aortic stent grafts (Fig. 34.3).
Beyond fenestrated, chimney, sandwich and branched graft configurations, additional techniques are also under development. Multilayer stents are semiporous and allow perfusion of visceral vessels through the graft wall while altering flow dynamics in the aneurysm sac, causing thrombosis of the sac. Sac-anchoring (Nellix, Endologix) and endostapling (HeliFX, Aptus Endosystems) technologies are also being developed.
When a patient with a juxtarenal aneurysm is being considered for a fenestrated graft, a CTA with multiplanar reconstructions is essential. Imaging from 2 cm above the diaphragm to below the symphysis pubis at 1-mm intervals is performed. Postprocessing software is essential as it allows for flexibility to manipulate planes in order to achieve greater accuracy and precision for measurements. Several software platforms exist for postprocessing including TeraRecon iNtuition, 3mensio Medical Imaging Vascular imaging software, and OsiriX MD.
Fenestrated device planning begins by determining where the proximal sealing stent will reside. The proximal infrarenal neck must be ≥4 mm and the total seal zone should be >15 mm from the proximal aneurysm edge. The bottom of the celiac artery is typically used as the reference point. Anatomical length measurements are made from the reference point to the middle and bottom of the SMA, middle of the highest and lowest renal arteries, start of the aneurysm, aortic bifurcation, and both of the iliac fixation sites.
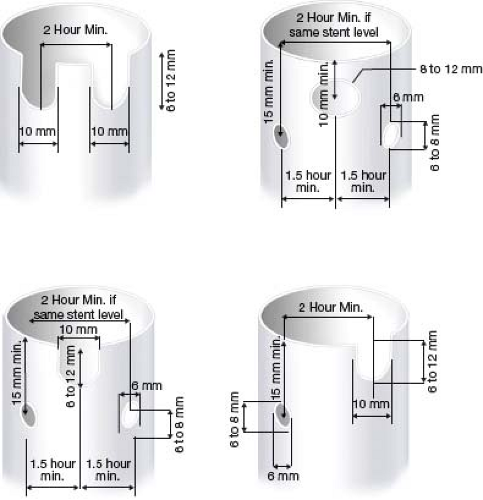
Determining the proximal edge of the graft to the bifurcation is next performed by measuring 15 mm proximal to the edge of the aneurysm. Assessment of the SMA or other visceral vessels within this seal zone is then noted and the fenestration configuration is determined. Some surgeons attempt to simplify the procedure by minimizing the number of vessels incorporated into the repair. However, our preference is to maximize the seal zone by starting with a full SMA scallop and then adding renal fenestrations. Measurements of the distances between visceral arteries, vessel diameters, and radial or clock positions of the visceral arteries are then performed. The diameter of the graft is typically chosen to be between 10% and 25% larger than the proximal implantation site. Scallops are used to accommodate more proximal vessels. Small fenestrations are generally used for renal and accessory renal arteries in the setting of a very short neck (<10 mm). Grafts may include no more than two of any one type of fenestration (small, large, and scallop). Fenestrations cannot be placed distal to the lowest sealing stent. In general, small fenestrations must be ≥1 hour 30 minutes away from a large fenestration and ≥2 hours apart from each other if on the same stent level. Two scallops or one scallop and one fenestration must be ≥2 hours apart. The most common graft configuration is an SMA scallop with two small fenestrations for the renal arteries (Fig. 34.4).
The inner aortic vessel diameter is measured at the level of the fenestrations which determine the lateral placement of the fenestrations in graft construction. Traditional EVAR measurements are also taken of the sealing zones and graft length. Proper alignment of fenestrations requires the ability to readily rotate the device. Thus, any relative narrowing within the proximal sealing area may limit the rotational ability unless the narrowest diameter is larger than the initial graft deployment with the diameter reducing ties in place. Additionally, access vessels must be evaluated with respect to tortuosity, diameter, stenosis, calcification, and preexisting stents as these may impact rotational ability as well. Distal implantation sites are oversized approximately 15% to 25%,
although in vessels with diameters greater than 16 mm greater additional oversizing may be considered.
although in vessels with diameters greater than 16 mm greater additional oversizing may be considered.
Vascular Access
Percutaneous access is our preferred method for obtaining common femoral artery (CFA) access, although a cutdown may also be done. For percutaneous access we prefer the needle entry into the artery be performed with ultrasound (US) guidance, which assists with precise placement of the puncture needle, ensures an anterior wall puncture, and allows the operator to note any disease within the CFA. US assists with identifying CFA disease which may alter the precise location of needle entry and be incorporated into the decision of using a Perclose device or cutting down at the conclusion of the procedure. Small, tortuous, or overly calcified iliac arteries may limit endograft delivery. An external iliac conduit may be created by open surgical anastomosis of a PTFE graft to the iliac artery, or an internal conduit may be created via the placement of a covered stent graft within the iliac artery with subsequent balloon dilation.
US-guided access begins with a transverse US probe to clearly identify the femoral bifurcation. Once the bifurcation is identified the probe is turned longitudinally on the artery and followed cephalad until the femoral head is located. A high puncture on the CFA increases the risk of retroperitoneal bleeding and a low puncture at the level of the SFA may predispose the patient to a high incidence of arteriovenous fistula, hematoma, and pseudoaneurysm formation.
A 21-gauge needle is visualized as it punctures only the anterior wall of the artery and insertion of a 0.018-in wire is performed. A quick fluoroscopic confirmation is performed to document the puncture site relative to the femoral head and confirm wire placement into the iliac artery and aorta. A 3-Fr microsheath is then placed followed by a 0.035-in wire. The needle tract is dilated with a 7-Fr introducer. Two Perclose devices are then deployed in a preclose fashion (fired but not cinched down), followed by placement of a 7-Fr sheath. This process is then repeated in the second CFA. Systemic heparin is then administered with a goal ACT of 250.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree