John Futchko, Katie MacCallum, and Aksim G. Rivera Department of Surgery (Vascular Surgery), Albert Einstein College of Medicine-Jacobi Medical Center, Bronx, NY, USA Since it was first described in 1986 [1], endovascular aneurysm repair (EVAR) has become the preferred treatment option for many patients with abdominal aortic aneurysms (AAAs). Multiple studies have shown lower 30‐day mortality rates and fewer perioperative complications as compared to open AAA repair [2–4]. Initial concerns regarding durability largely have been alleviated by stent‐graft redesign and follow‐up trials examining long‐term outcomes [5–7]. While overall complication rates are favorable, reintervention rates remain significantly higher than open repair [8]. EVAR requires extensive preoperative planning including appropriate patient selection, detailed imaging, measurements, and graft selection. There are various intraoperative pitfalls, such as vascular access and endoleaks, which the surgeon must be able to identify and troubleshoot. This chapter will provide an overview of the steps of the procedure as well as management of potential issues that may arise. Though EVAR is appropriate for most patients, including those otherwise deemed too high‐risk for open repair [9], it is not “one size fits all.” Various criteria should be considered, particularly anatomic suitability. One advantage of EVAR over open repair is that it may be performed under anesthesia suited to the patient’s needs. General anesthesia is commonly used; however, either regional (spinal or epidural) or local anesthetic with sedation may be chosen depending on the individual’s comorbidities and type of access to be performed. This gives the anesthesiologist relative flexibility as compared to open repair which mandates general anesthesia. Potential anatomic challenges include a short or severely angled aortic neck, circumferential thrombus, and iliac artery disease, but none of these alone is an absolute contraindication [10–12], particularly with advancements in graft design. Most surgeons consider the character of the neck to be the most important anatomic factor with length, diameter, angulation, and shape all playing important roles in the seal of the proximal graft. Pitfalls related to poor distal vessel quality will be addressed later in the section on vascular access. Preoperative imaging is essential to identifying these issues. All patients undergoing EVAR will require preoperative imaging. Computed tomography arteriography (CTA) with three‐dimensional (3D) reconstructions is the most common and the most useful for planning. Multiple software packages are available, including open source platforms, which enable the surgeon to create precise measurements accounting for the angulation and rotation of the blood vessels involved through centerline reconstructions (Figure 5.1). Figure 5.1 Pictured on the left is a traditional CTA coronal view of an infrarenal aneurysm. To the right is 3D reconstruction using available software. Such imaging modalities allow for accurate sizing and pre‐operative case planning. As proper CTA requires intravenous contrast administration, it should be performed judiciously in those patients with chronic kidney disease or concern for contrast allergy. For those who are unable to receive contrast dye, noncontrast may be performed. Unfortunately, this will lead to a subpar understanding of the patient’s anatomy. Distal occlusive disease or the presence of laminated thrombus may not be appreciated. In these patients, alternative imaging may also be considered such as carbon dioxide angiography [13] or intravascular ultrasound (IVUS). IVUS has also emerged as an essential intraoperative adjunct in performing EVAR for all patients [14]. Once the surgeon has decided EVAR is a suitable option for an individual patient, the next crucial step is selection of the stent‐graft itself. There are multiple companies with devices currently approved for use in the United States and even more in clinical trials. In choosing a graft, one must consider the following key components: profile, graft material, stent material, modularity, ease of use, and range of treatable aortic neck and iliac artery. The delivery system profile is of particular importance in an aging population or in female patients who may have smaller femoral vessels, thus limiting access. At the time of writing, the smallest delivery sheath system is the Alto™ system (Endologix, Irvine, CA, USA) with an outside diameter (OD) of 15 Fr. All commercially available grafts are currently made with either woven polyester or polytetrafluroethylene (PTFE), similar to materials used during open surgical repair. Proximal fixation may be either infrarenal or suprarenal depending on the inclusion of a bare‐metal stent component. Positive fixation may be achieved with additional features such as metal hooks, barbs, anchors, or staples. Additional graft features, such as column support and radial force, will assist with fixation and, ideally, prevent caudal migration over the life of the device. Manufacturers have selected to produce either bi‐ or tri‐modular devices, with three‐piece grafts consisting of the main body and individual iliac limbs as opposed to a main body and iliac piece with a separate contralateral limb piece. Graft selection depends not only on patient anatomy but also on the operator’s comfort with the system. Each stent‐graft has unique features and thus requires specifically tailored measurements. Most manufacturers have worksheets available to assist in sizing. Generally speaking, grafts should be oversized approximately 10–20% in comparison to the diameter of the aortic neck and iliac arteries to minimize the risk of graft complications, including graft migration and both early and late endoleak during aortic remodeling. While each available endograft device has its own specific instructions for use (IFU), there are several general guidelines in sizing, which can help to determine the complexity of repair required dependent on individual patient anatomy. One of the most crucial initial measurements in endograft sizing is the distance between the lowest renal artery and the most proximal portion of aneurysmal aorta, typically referred to as the “neck length.” This segment of aorta acts as the proximal fixation point for the sealing stents found at the proximal ends of most endograft devices. While there is some variation, as some grafts, such as the Zenith™ (Cook Medical, Bloomington, IN, USA), have bare‐metal suprarenal fixation stents, these sealing zone stents provide the necessary wall apposition that leads to aneurysm exclusion. Equally as important is the quality of this aortic segment, as the presence of even mild aneurysmal degeneration or thrombus can lead to intraoperative embolic events, endoleak, or improper aortic remodeling. In general, an aortic neck of at least 15 mm is required for on‐label use of most commercially available endografts. However, there are two available devices, the Endurant™ (Medtronic, Minneapolis, MN, USA) graft and Alto graft, which have specific IFU for aneurysms with less than 10 mm of available neck. In the case of the Endurant graft, the use of endoanchors improves apposition and fixation and allows for deployment with as little as 4 mm of available neck length. The measurement of the aortic neck diameter is essential to prevent repair failure and thus equally as important in endograft sizing as neck length. It is typically taken 15 mm from the lowest renal and involves a wall‐to‐wall measurement in the axial cuts in a plane perpendicular to the course of the aortic lumen; although this may vary based on device. Most device manufacturers recommend oversizing by 10–20% of the aortic diameter. In doing so, current EVAR devices can accommodate aortic diameters between 18 and 32 mm. Oversizing assures adequate wall apposition and reduction of the risk of a type I endoleak. However, overzealous oversizing can result in graft pleating of the fabric and type I endoleak, as well as an increase in late graft migration and aortic neck dilation. This is also important to remember in patients with conical aortic necks. In such cases, most recommend taking the average of both diameters to determine a final endograft stent size. In cases where aneurysmal degeneration extends into the visceral segment and the patient is not suited to open repair, there are several endovascular options falling into three general categories: fenestrated endografts, parallel grafts, and debranching procedures. Fenestrated endografts have now become commercially available (Zenith). Tailored to an individual patient’s anatomy, these custom‐made endografts allow for endovascular repair of juxtarenal aneurysms through creation of windows in the endograft designed to align with aortic branch vessels allowing for perfusion (and possible stenting) of visceral arteries. These devices have their own anatomic limitations and offer only limited treatment options in “off‐the‐shelf” emergent repair situations. When such devices cannot be utilized, alternative complex endovascular techniques allow for appropriate repair and coverage. Parallel stent‐grafts, which include snorkels, periscopes, and chimneys, refer to stent‐grafts placed in parallel alongside aortic endografts to allow for perfusion of branch vessels that would otherwise be covered. These repairs are often complicated with endoleak between stent‐grafts, so called “gutterleaks,” as well as stent‐graft thrombosis and type I endoleaks. Debranching refers to open surgical debranching and bypass of visceral vessels (i.e. celiac, SMA, and renal arteries) to alternative arterial inflow targets (typically iliac arteries) prior to planned endograft coverage. Calculating the aortic length to repair requires several measurements: the lowest renal artery to the aortic bifurcation, the bifurcation to the right hypogastric artery, and the bifurcation to the left hypogastric artery. These measurements can prove challenging, especially in the case of a tortuous aorta or iliac arteries; however, this can be aided with the use of centerline reconstruction software. For most devices appropriate wall apposition requires at least 10 mm of distance between the aortic bifurcation and the internal iliac artery. The need for longer iliac limbs typically occurs in patients with more tortuous iliacs. Patients with splayed aortic bifurcations may benefit from “balleting” (crossing) the iliac limbs, which also requires longer limb lengths (Figure 5.2). The distal seal zone along the iliac vessels also plays a critical role in graft sizing. As with proximal fixation, distal fixation typically requires 10–20% oversizing of the measured iliac diameter and is crucial to prevent type IB endoleaks, iliac aneurysmal degeneration, or iliac thrombosis. Iliac arteries less than 7 mm or greater than 25 mm may render endovascular treatment unsuitable. For patients with concurrent iliac occlusive disease, treatment of such lesions should occur prior to endograft placement, as these aortic endografts do not provide the radial force necessary for treatment of stenotic lesions. Figure 5.2 In cases where aortic anatomy is not suitable for on‐label device use, graft alteration or “physician‐modified endografts” as pictured above can provide an endovascular solution in patients who are otherwise poor candidates for difficult open repairs. For patients with concurrent aneurysmal iliac disease, the Iliac Branch Excluder™ Device (W.L. Gore, Flagstaff, AZ, USA) allows for canalization and preservation of the iliac branch vessels, with exclusion of associated aneurysm. These devices are especially important in patients with occluded contralateral hypogastric vessels. Ultrasound‐guided percutaneous access has become increasingly routine in both EVAR and thoracic endovascular aortic repair
5
Endovascular Abdominal Aortic Aneurysm Repair (EVAR)
Introduction
Patient Selection
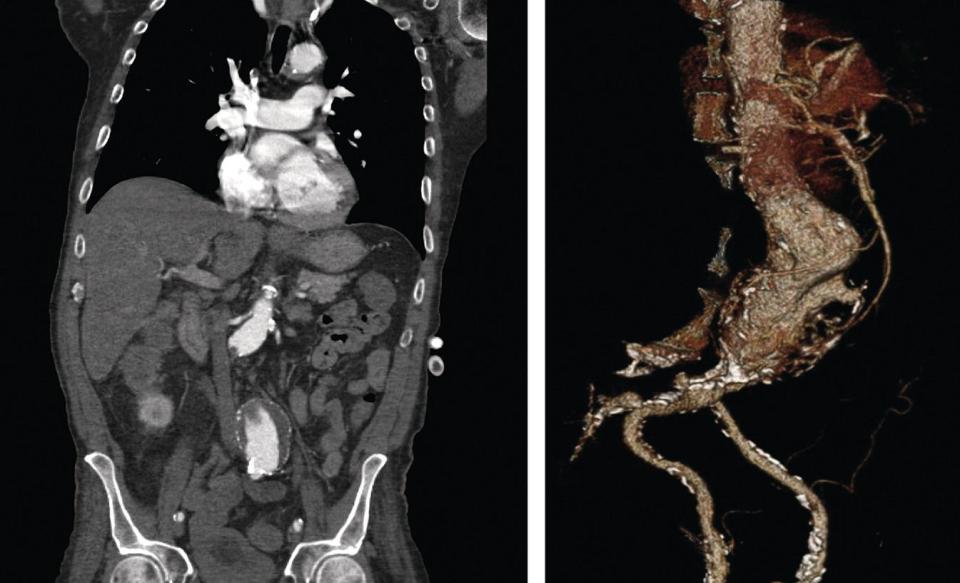
Preoperative Imaging and Measurements
Graft Selection
Graft Sizing
Neck Length
Neck Diameter
Branch Vessels
Aortic Length Measurements

Step 1. Vascular Access
Percutaneous
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


