Fig. 14.1
Effect of bosentan on the primary endpoint of change in 6-min walk distance at week 16 from the BREATHE-1 trial. Based on a placebo-corrected improvement in walk distance of 44 m, and its safety profile, bosentan was approved at a dose of 125 mg twice daily (From [16])
In addition to the above “registration” trials of bosentan, a randomized controlled trial of bosentan in PAH patients less functionally impaired (WHO class II), the EARLY trial, demonstrated a benefit in reducing PVR and preventing clinical worsening at 6 months. No statistically significant effect on 6MWD was seen, although baseline walk distance was greater than 400 m, confirming that this cohort had better baseline exercise capacity [17].
Long‐term survival data in patients on bosentan, although uncontrolled, have been published. Of the 169 IPAH patients enrolled in the two pivotal trials of bosentan, estimated survival at 1 year and 2 years was 96 % and 89 % respectively, as compared to the predicted survival of 69 % and 57 % [18] (based on a validated NIH equation calculating predicted survival from baseline hemodynamics). It should be acknowledged that there are no prospective controlled survival data with the newer agents, given obvious ethical concerns about such trials in the era of existing therapy.
Bosentan is primarily metabolized in the liver through the P450 enzyme system. Clinical trials have shown that bosentan can precipitate hepatocellular injury, in particular when given at higher doses. Combined data from multiple trials have shown that there is a greater incidence in hepatic injury in patients treated with bosentan when compared to placebo. Greater than threefold elevations in aminotransferases were seen in 11 % of bosentan-treated patients (n = 658) compared with 2 % of patients treated with placebo (n = 280).
In the BREATHE-1 study, increases in hepatic aminotransferases occurred in 10 % of the patients and were found to be dose-dependent (more frequent in the 250 mg group), and reversible with dose reductions or upon stopping the drug. Based on these findings, the recommended dose of bosentan is 125 mg twice daily. Patients on bosentan must undergo baseline monitoring of liver function tests prior to initiation of the drug, and monthly thereafter.
Bosentan in Other Types of Pulmonary Hypertension
The initial bosentan trials only included adult patients with IPAH and associated PAH, predominantly scleroderma or connective tissue-related PAH. Data regarding the use of bosentan in other classes of PAH are limited. BREATHE-4 was a small, uncontrolled, prospective study of 16 patients with class III and IV HIV-associated PAH. The study found that HIV-associated PAH patients treated with bosentan had similar safety and efficacy profiles to prior groups studied [19]. At 16 weeks there were significant improvements in 6MWD, echocardiographic parameters, and quality of life scores. However, there was no comparison placebo arm. There was also an improvement in functional class, with 14 out of the 16 patients improving at least one class when compared to baseline. Treatment with bosentan did not have a negative impact on control of HIV infection. In this small study, bosentan had similar hepatic tolerability to that found in other patients with PAH, despite several patients being co-infected with either hepatitis B or C virus. In patients with hepatitis co-infection, bosentan should be used with caution and frequent monitoring of liver function tests.
BREATHE-5 was a randomized, placebo-controlled trial that evaluated the effect of bosentan in patients with Eisenmenger’s syndrome due to congenital heart disease and functional class III PAH [20]. Fifty-four patients were randomized in a 2:1 fashion to bosentan (n = 37) or placebo (n = 17) for 16 weeks. Bosentan treatment did not reduce systemic arterial blood oxygen saturation, and showed significant improvement in hemodynamics (PVR, mPAP) and exercise capacity, with a treatment effect of +53 m in 6MWD. An open-label extension of this study revealed improvement in functional class in 24 out of the 37 patients. A subgroup analysis comparing atrial septal defect to ventricular septal defect patients receiving bosentan did not show any significant difference between the two groups [21]. This suggests the location of septal defects is not an important determinant of treatment response with bosentan.
The Bosentan Effects in iNopErable Forms of chronic Thromboembolic pulmonary hypertension (BENEFiT) trial investigated bosentan use in CTEPH [22]. One hundred and fifty-seven patients with CTEPH, who had either inoperable disease or persistent pulmonary hypertension 6 months after pulmonary endarterectomy, were enrolled. A statistically significant treatment effect of −24.1 % reduction in PVR was demonstrated in patients treated with bosentan over placebo. There was an improvement in 6MWD, but it did not reach statistical significance.
Ambrisentan
Ambrisentan is a specific ETA receptor antagonist approved for PAH, functional classes II and III at doses of 5 or 10 mg once daily. Following a Phase 2, dosing study showing improvement in pulmonary hemodynamics [23], two randomized controlled trials, ARIES 1 and ARIES 2 (ARIES 1: 5 mg, 10 mg, placebo; ARIES 2: 2.5 mg, 5 mg, placebo) were conducted, enrolling a total of 394 patients [24]. Both trials demonstrated improvement in the primary endpoint of placebo-corrected 6MWD (Fig. 14.2). In ARIES-2, there was a significant improvement in time to clinical worsening in the treatment group as compared with placebo. There was a trend toward improvement in time to clinical worsening in the ARIES-1 study, but it was not statistically significant (p = 0.307). World Health Organization (WHO) functional class improvement was significant in ARIES-1 and there was trend toward improvement in ARIES-2 but did not reach statistical significance (p = 0.117). Two hundred and ninety-eight patients were enrolled and followed in a long-term extension study over 48 weeks. Eighteen patients required additional therapies (prostanoids or phosphodiesterase type-5 [PDE-5] inhibitors). Of the 280 patients continued on ambrisentan monotherapy, the improvement in 6MWD at 12 weeks was 40 m and maintained at 39 m. Although there were no patients with elevations in serum aminotransferases >3 times upper limit of normal while on ambrisentan, in the trials, long-term follow-up has revealed cases of transaminase elevations which resolve upon discontinuation of ambrisentan. However, because post-marketing surveillance suggested a minimal risk of drug-induced liver toxicity, the FDA removed the black box warning and requirement for monthly liver function monitoring. However, regular monitoring of pregnancy tests and hemoglobin is still required.
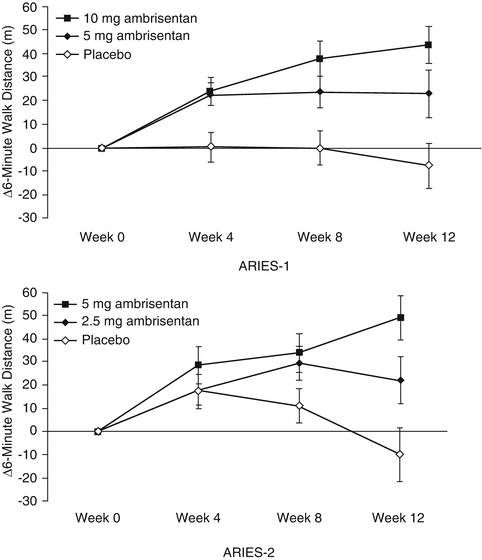

Fig. 14.2
Effect of ambrisentan on the primary endpoint of change in 6-min walk distance at week 12, from the ARIES-1 and ARIES-2 trials. Based on this finding, ambrisentan was approved at doses of 5 mg daily and 10 mg daily (From [24])
Macitentan
Macitentan is a novel dual ERA that was developed by modifying the chemical structure of bosentan to produce a drug with high oral efficacy and lipophilicity. This led to discovery of several alkyl sulfamide-substituted pyrimidines, including macitentan. Chemically, macitentan is N-[5-(4-bromophenyl)-6-[2-[(5-bromo-2-pyrimidinyl)oxy]ethoxy]-4-pyrimidinyl]-N′-propylsulfamide [25].
Compared with bosentan and ambrisentan, macitentan has a higher pKa and distribution coefficient, resulting in a higher percentage of nonionized drug at physiologic pH and favoring distribution across the phospholipid bilayer of the cell membrane. Compared to bosentan, macitentan also has improved receptor association and dissociation kinetics with more sustained receptor binding [26]. In human pulmonary artery smooth muscle cells, this leads to insurmountable antagonism and suggests that macitentan may have the ability to block endothelin more effectively in the setting of variable endothelin levels.
Macitentan: Initial Phase 1 and 2 Clinical Trials
Macitentan was first studied in healthy subjects in single dose followed by ascending multiple dose studies of 1–30 mg of macitentan versus placebo [27]. In these studies, macitentan was well tolerated and pharmacokinetics were supportive of once daily dosing with a maximal effect on endothelin levels at a dose of 10 mg. The half-life of macitentan was 14–18 h, while the half-life of ACT-132577, an active but less potent metabolite, was approximately 48 h. Drug elimination and formation of the active metabolite is catalyzed by cytochrome (CYP) P450, predominantly CYP3A4 and CYP2C19.
Macitentan: Phase 3 Clinical Trial
The landmark Phase 3 clinical trial that led to approval of macitentan in the U.S. and several other countries was the SERAPHIN (Study with an Endothelin Receptor Antagonist in Pulmonary arterial Hypertension to Improve cliNical outcomes) trial [28]. The study was designed as an event-driven morbidity and mortality trial. In this study, 742 patients with symptomatic PAH (52 % FC II, 46 % FC III and 2 % FC IV) were randomized 1:1:1 to receive placebo (n = 250), macitentan 3 mg (n = 250), or macitentan 10 mg (n = 242) and were followed for a median duration of 115 weeks. The study cohort included patients with idiopathic and heritable PAH (57 %), PAH associated with connective tissue disease (31 %), PAH associated with congenital heart disease (8 %), and PAH associated with HIV or drug/toxin exposure (4 %). Sixty-four percent of patients were on stable background PAH therapy with phosphodiesterase inhibitors or oral or inhaled prostanoids which they were allowed to continue throughout the study.
The primary endpoint was time from initiation of treatment to the first occurrence of a morbidity event related to PAH or death from any cause in an intention-to-treat analysis. The components of the morbidity/mortality event endpoint included death, atrial septostomy, lung transplantation, initiation of intravenous or subcutaneous prostanoids, or a composite endpoint for worsening of PAH.
The primary endpoint occurred in 46.4 (n = 116), 38 (n = 95), and 31.4 % (n = 76) of patients treated with placebo, macitentan 3 mg and macitentan 10 mg, respectively, with a hazard ratio of 0.70 (97.5 % CI, 0.52–0.96; p = 0.01) for macitentan 3 mg versus placebo and a hazard ratio of 0.55 (97.5 % CI 0.39–0.76; p < 0.001) for macitentan 10 mg versus placebo (Fig. 14.3). Worsening of PAH was the most frequent first event, which is not unexpected since worsening of PAH often precedes other components of the endpoint, such as initiation of prostanoids or mortality.
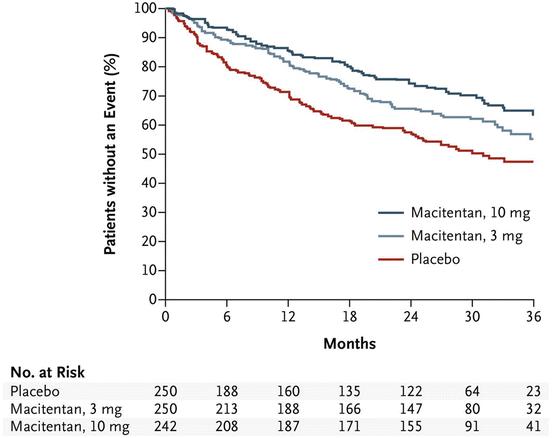

Fig. 14.3
Effects of macitentan compared to placebo on time to first morbidity/mortality event. The hazard ratio for macitentan 10 mg daily compared to placebo was 0.55 (relative risk reduction of 45 % over the course of the study). Based on this finding, macitentan was approved at 10 mg daily (From [28])
A prespecified secondary endpoint, death or hospitalization due to PAH, occurred in 33.6 % of the placebo group, 26 % of the macitentan 3 mg group and 20.7 % of the macitentan 10 mg group with a hazard ratio of 0.67 (97.5 % CI, 0.46–0.97, p = 0.01) for macitentan 3 mg versus placebo and a hazard ratio of 0.50 (97.5 % CI 0.34–0.75, p < 0.001) for macitentan 10 mg versus placebo (Fig. 14.3). Hospitalization accounted for the majority of these events, and there was no statistically significant difference in mortality between the groups. The study, however, was not designed or powered to show an effect on the endpoint of mortality alone.
The benefit of macitentan was observed irrespective of background therapy for PAH; macitentan 10 mg reduced the risk of the primary endpoint by 38 % (95 % CI 11–57 %) in the presence of background PAH therapy and 55 % (95 % CI 28–72 %) in the absence of PAH background therapy. Macitentan also demonstrated an improvement in secondary endpoints, including functional class and exercise capacity. In a subset of 145 patients who had right heart catheterizations and pulmonary hemodynamics reported at baseline and month 6, patients in both macitentan groups also had significant decreases in PVR (66.4 % [95 % CI 56.6–77.8 %] and 61.5 % [95 % CI 62.4–81.4 %] of placebo-corrected change from baseline for 3 mg and 10 mg macitentan, respectively) and an increase in cardiac index (0.69 L/min/m2 [95 % CI 0.40–0.97] and 0.63 L/min/m2 [95 % CI 0.28–0.97] placebo-corrected change from baseline for 3 mg and 10 mg macitentan, respectively).
SERAPHIN was the first event-driven study ever done in PAH. Compared to other clinical trials that led to drug approval for ambrisentan and bosentan in which patients were only exposed to therapy for 12–16 weeks [15, 16, 24], the treated patients in SERAPHIN had a median drug exposure of 115 weeks. Additionally, compared to other studies that have predominantly evaluated PAH monotherapy versus placebo, this study also included patients on background PAH therapy and demonstrated a beneficial effect of macitentan as monotherapy or in combination with other PAH medications. It is also important to note that even in patients on background PAH therapy, there was a significant incidence of morbidity/mortality over the course of the study, which was decreased with macitentan.
In SERAPHIN, the occurrence of aminotransferase elevation or edema and the number of people discontinuing treatment due to adverse effects were similar in placebo and active treatment groups. There was a small, dose-related decrease in hemoglobin from macitentan. Based on the results of SERAPHIN, macitentan (Opsumit, Actelion Pharmaceuticals) was approved by the United States FDA in October 2013 for the treatment of patients with Group 1 PAH and functional class II–IV symptoms at a recommended dose of 10 mg daily. It became commercially available for use in the United States in November 2013. Similar to ambrisentan, there is no required monthly liver function test monitoring for macitentan.
Comparing ERAs to Other PAH Therapy
ERAs have rarely been compared to other PAH therapies in head-to-head trials. The Sildenafil versus Endothelin Receptor Antagonist for Pulmonary Hypertension (SERAPH) trial directly compared bosentan to sildenafil. Twenty-six patients with class III PAH (IPAH and associated with connective tissue disease) were randomized to receive either sildenafil (50 mg b.i.d. for 4 weeks followed by 50 mg t.i.d.) or bosentan (62.5 mg b.i.d. for 4 weeks followed by 125 mg b.i.d.) as initial PAH monotherapy [29]. After 16 weeks, there was not a significant difference between the two groups in 6MWD, right ventricular mass, cardiac function, brain natriuretic peptide, or Borg dyspnea score.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


