Chapter 2 Endoscopic Therapy of Endobronchial Typical Carcinoid
Case Description
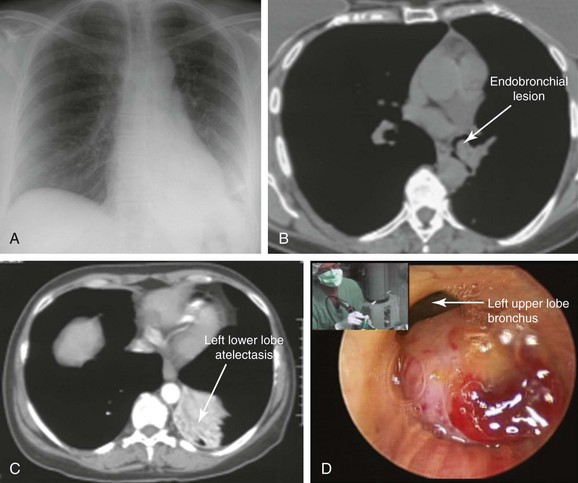
A 47-year-old woman was hospitalized at an outside institution for hemoptysis and left lower lobe pneumonia. She had a history of wheezing for several months, which was unresponsive to bronchodilators and inhaled corticosteroids. Spirometry was normal. Three weeks before admission, she developed cough and fever. When she developed hemoptysis (half a cup of bright red blood within several hours), she presented to the emergency department. Vital signs showed a temperature of 38.5° C, heart rate of 120 bpm, respiratory rate of 28/min, and blood pressure of 150/75. She had no pain. Wheezing was noted on auscultation of the left hemithorax, and the patient had diminished air entry at the left base. The rest of the examination was unremarkable. Laboratory tests were normal except for a white blood cell count of 24,000/mm3. Chest radiography showed left lower lobe opacification (Figure 2-1, A). Computed tomography (CT) scan revealed left lower lobe atelectasis and a distal left main bronchial mass completely obstructing the left lower lobe bronchus and partially obstructing the entrance to the left upper lobe (see Figure 2-1, B). The patient was started on broad-spectrum antibiotics for pneumonia. Flexible bronchoscopy showed an exophytic endoluminal hypervascular distal left main bronchial lesion. Endobronchial biopsies revealed typical carcinoid.*
After bronchoscopy, hemoptysis increased, prompting transfer to our institution for palliation and bronchoscopic resection of the endobronchial tumor. Rigid bronchoscopy under general anesthesia confirmed the flexible bronchoscopic findings (see Figure 2-1). Neodymium-doped yttrium aluminium garnet (Nd : YAG) laser–assisted resection was performed to restore airway patency (Figure 2-2).
Discussion Points
1. Describe two strategies to decrease the risk of bleeding from bronchoscopic biopsy of this tumor.
2. Discuss the role of imaging studies in diagnosis and staging of carcinoid tumors.
3. List three open surgical treatment alternatives for endobronchial carcinoid.
4. Describe how tumor histology and morphology affect treatment decisions for carcinoid tumors.
Case Resolution
Initial Evaluations
Physical Examination, Complementary Tests, and Functional Status Assessment
Symptoms in patients with bronchial carcinoid depend on the tumor size, site, and growth pattern. For instance, a small peripheral carcinoid may be an incidental finding, and a large central tumor may result in symptoms similar to those found in our patient: cough, hemoptysis (due to its hypervascularity), and obstructive pneumonia. Some patients may also have shortness of breath.1 The diagnosis is often delayed, and patients may receive several courses of antibiotics, bronchodilators, or inhaled corticosteroids to treat recurrent pneumonia or suspected asthma. In one study, 14% of patients had been treated for asthma for up to 3 years before the tumor was discovered.2 The wheezing noted in our patient was localized to the left hemithorax, reflecting focal airway obstruction, not bronchoconstriction. In fact, diffuse wheezing is rare in patients with carcinoids, regardless of tumor location, because only 1% to 5% of patients exhibit hormone-related symptoms such as carcinoid syndrome.† In part, this reflects the low incidence of hepatic metastases—2% and 5%, respectively—for typical and atypical carcinoids.2,3 In the setting of liver metastasis, however, more than 80% of patients have symptoms of carcinoid syndrome.1 Bronchial and other extraintestinal carcinoids, whose bioactive products are not immediately cleared by the liver, may cause the syndrome in the absence of liver metastasis because of their direct access to the systemic circulation. However, bronchial carcinoids have low serotonin content because they often lack aromatic amino acid decarboxylase and cannot produce serotonin and its metabolites; they only occasionally secrete bioactive amines. Elevated plasma or urinary secretory product levels such as 5′-hydroxyindoleacetic acid (5′-HIAA) thus are rarely detected. Elevation of plasma chromogranin A* is a relatively sensitive (≈75%) marker of bronchopulmonary carcinoids, but elevated levels are also seen in approximately 60% of patients with small cell carcinoma, and false-positive elevations can occur in renal impairment, in atrophic gastritis, and during proton pump inhibitor therapy.4 Measurement of its levels is considered useful only in following disease activity in the setting of advanced or metastatic carcinoid.5 No such serologic or urinary testing was performed in our patient before bronchoscopic intervention was provided.
With regard to radiographic studies, when the tumor is centrally located, as in our patient, bronchial obstruction may occur and atelectasis is noted (see Figure 2-1, C). Compared with chest x-ray, CT provides better resolution of tumor extent and location, as well as the presence or absence of mediastinal lymphadenopathy. High-resolution CT allows characterization of centrally located carcinoids, which may be purely intraluminal, exclusively extraluminal, or, more frequently, a mixture of intraluminal and extraluminal components (the “iceberg” lesion). In the setting of post obstructive pneumonia, a clear distinction between intraluminal and extraluminal extension can be properly made only after post obstructive debris and atelectasis have been removed.6 Tumor morphology impacts management because bronchoscopic treatments alone are considered by some investigators to be acceptable therapeutic alternatives for purely intraluminal typical carcinoids.6 Chest CT scan should be performed as part of nodal staging for carcinoid tumors. Atypical carcinoids have a higher recurrence rate and present more often with hilar or mediastinal nodal metastases (20% to 60% vs. 4% to 27%), when compared with typical carcinoids.1 This patient had no evidence of mediastinal or hilar lymphadenopathy on CT scan; because atelectasis and pneumonia were present, it was unclear whether the tumor had an extraluminal component (e.g., mixed obstruction, iceberg lesion).
The bronchoscopic appearance of this patient’s carcinoid is classic: a pink to red vascular mass attached to the bronchus by a broad base. In one study, 41% of patients presented with evidence of bronchial obstruction.3 These lesions occasionally can create a ball-valve effect (see video on ExpertConsult.com) (Video I.2.1![]() ). The hypervascular pattern has raised concern for bleeding following bronchoscopic biopsy. Some authors report bleeding in a quarter to two thirds of their patients, and some advise against biopsy when carcinoid is suspected.7–9 In a review of 587 biopsies by flexible and rigid bronchoscopy, significant hemorrhage was seen in 15 (2.6%) patients, but 11 (1.9%) of these patients did not require transfusion or emergency surgery. In four patients (0.7%), emergency thoracotomy was necessary to address the problem of massive uncontrollable hemorrhage.10 Other authors showed that biopsy is safe, significantly increases the diagnostic yield, and is not associated with significant hemorrhage.3,11 It is wise to have electrocautery, argon plasma coagulation, or a laser readily available to control spontaneous or post biopsy hemorrhage if necessary (see video on ExpertConsult.com) (Video I.2.2
). The hypervascular pattern has raised concern for bleeding following bronchoscopic biopsy. Some authors report bleeding in a quarter to two thirds of their patients, and some advise against biopsy when carcinoid is suspected.7–9 In a review of 587 biopsies by flexible and rigid bronchoscopy, significant hemorrhage was seen in 15 (2.6%) patients, but 11 (1.9%) of these patients did not require transfusion or emergency surgery. In four patients (0.7%), emergency thoracotomy was necessary to address the problem of massive uncontrollable hemorrhage.10 Other authors showed that biopsy is safe, significantly increases the diagnostic yield, and is not associated with significant hemorrhage.3,11 It is wise to have electrocautery, argon plasma coagulation, or a laser readily available to control spontaneous or post biopsy hemorrhage if necessary (see video on ExpertConsult.com) (Video I.2.2![]() ).
).
Comorbidities
This patient had no signs or symptoms suggesting hormone-related disorders caused by carcinoid, such as Cushing’s syndrome, acromegaly, or typical or atypical carcinoid syndrome.*
Patient Preferences and Expectations
The patient had expressed fears of having lung cancer. She wanted clarification in terms of her diagnosis, prognosis, and follow-up after treatment. It was explained to her that bronchial carcinoid tumors are rare lung malignancies that rarely spread outside the chest.*12,13 We were aware of data showing that the incidence of distant metastases at diagnosis for bronchopulmonary carcinoids is 5.5%14; that overall, 27.5% of bronchopulmonary carcinoids exhibit invasive growth or metastatic spread; and that the overall 5-year survival rate is 73.5%. Therefore we did not use the term benign tumor in our conversation with the patient.12 Typical and atypical carcinoids, however, have different biologic behaviors and prognoses, causing them to be considered and reported as different tumors.2 Typical carcinoids, as seen in this patient, usually have a good prognosis, with 5 year survival of 87% to 89%. Distant metastases from typical carcinoids may occur in approximately 10% of patients, even many years after radical resection of the primary tumor. Prolonged 10 year follow-up is therefore recommended. Atypical carcinoids are associated with 5 year survival of 44% to 78%.1
Procedural Strategies
Indications
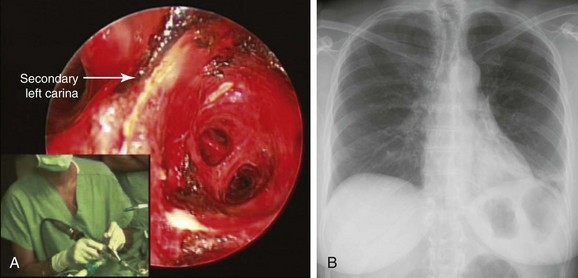
1. To palliate central airway obstruction in patients who are poor surgical candidates (Figure 2-3).15 Our patient, although considered operable (normal spirometry and no comorbidities), was not a good candidate for open surgery at the time of admission, given her ongoing sepsis from post obstructive pneumonia.
2. To guide open surgical procedures after laser bronchoscopic removal of an obstructing lesion.16 Investigators report that lung-preserving resections are facilitated by preoperative laser treatment in 10% to 12% of patients with central obstructing carcinoids. Treatment of airway obstruction before surgery allows the operator to estimate the extent of bronchoplastic surgery.16,17
3. As a reasonable alternative to immediate surgical resection in patients who present with an exophytic intraluminal tumor, good visualization of the distal tumor margin, and no evidence of bronchial wall involvement or suspicious lymphadenopathy by high-resolution CT. Close post-treatment follow-up is an integral component of such treatment.6
Expected Results
Bronchoscopic resection alone may provide prolonged recurrence-free survival for highly selected patients with a purely exophytic endoluminal bronchial carcinoid.6,18–21 These patients present with a polypoid (exophytic) intraluminal tumor, good visualization of the distal tumor margin (usually less than 2 cm in extent), and no evidence of bronchial wall involvement or suspicious lymphadenopathy by high-resolution CT. In the largest series of 72 patients treated with this approach (57 typical and 15 atypical carcinoids), initial bronchoscopic management resulted in complete tumor eradication in 33 patients (46%). Surgery was required in 37 patients (including 11 of the 15 with atypical carcinoids)—2 for delayed recurrence at 9 and 10 years. At a median follow-up of 65 months, 66 patients (92%) remained alive, and only 1 of the deaths was tumor related.6
Team Experience
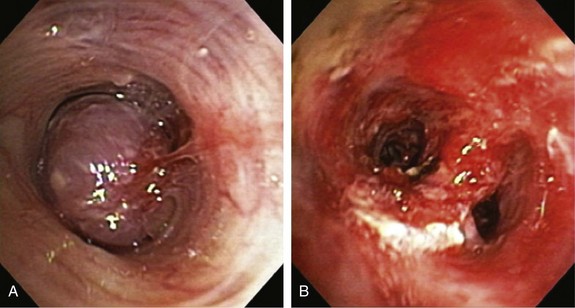
Rigid bronchoscopy with Nd : YAG laser resection is frequently performed in our referral center by a team of doctors and nurses experienced in managing critical central airway obstruction and hemoptysis. The goal of the operating team when this type of laser is used should be to remove unwanted tissue (tumor) with adequate hemostasis and minimum destruction of adjacent healthy tissue. Accurate removal of diseased tissue depends on the surgeon’s ability to visualize tissues and feel and control the shape and size of target tissues in three dimensions. Thus experience is important because complete and clear visualization of the treated area, ensuring hemostasis and minimization of adjacent laser-induced thermal injury, requires skillful operation of the rigid bronchoscope and knowledge of laser physics and laser-tissue interaction. For example, in a patient with mid-left mainstem bronchial carcinoid (Figure 2-4), the goal is precise removal of the lesion with minimal thermal trauma to the normal adjacent mucosa. Resection may be achieved in ways other than vaporization or widespread coagulation of blood vessels. Power density should be employed in ways that avoid damaging surrounding tissues to minimize interference, with consideration for future bronchoplastic procedures. If the tumor is completely removed, some investigators have suggested applying low-power density laser energy to residual bronchial surfaces to ablate and presumably kill residual tumor cells. Deep mucosal biopsies are warranted after a complete resection to ascertain the absence of disease.
Risk-Benefit Analysis
Open surgical resection is the preferred treatment approach for patients whose overall medical condition and pulmonary reserve will tolerate it. For patients whose condition does not permit complete resection, and for exceptional cases in which the lesion is entirely intraluminal, bronchoscopic resection may be an alternative.6 Our patient had post obstructive pneumonia, so we opted for initial bronchoscopic treatment to restore airway patency to the left lower lobe.
Therapeutic Alternatives
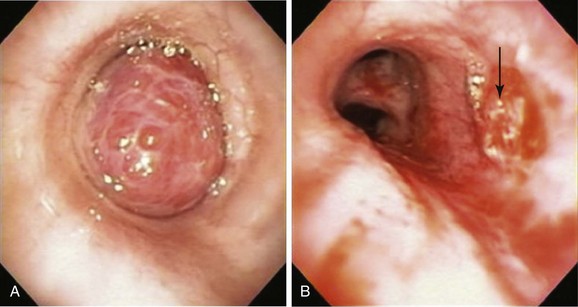
1. Surgical resection with complete mediastinal lymph node dissection is, in general, considered the treatment of choice for carcinoid tumor because it offers a real chance of cure. The goal is en bloc resection of the entire neoplasm (Figure 2-5) with preservation of functional pulmonary parenchyma if possible. Attempts to preserve lung parenchyma through the use of bronchoplastic techniques (e.g., sleeve, wedge, flap resection) to avoid lobectomy, bilobectomy, or pneumonectomy are justified and safe.22–24 Parenchyma-sparing operations based on bronchoplastic or sleeve resection do not alter the oncologic result and lead to a better quality of life.25 These procedures are possible only in the absence of “iceberg” lesions (in which the tumor appears entirely intraluminal bronchoscopically but has a significant extraluminal component that is evident with high-resolution computed tomography [HRCT]). The following surgical principles are recommended for bronchial carcinoids:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree