(1)
Department of Surgery, Columbia University College of Physicians and Surgeons, The Valley Columbia Heart Center, The Valley Hospital, Ridgewood, NJ, USA
(2)
Division of Pediatric Cardiothoracic Surgery, University of Utah, 100 N. Mario Capecchi Dr., Salt Lake City, UT 84113, USA
(3)
UP School of Medicine, University of Pennsylvania, Phi Building Suite 2A, 51 N. 39th St., Philadelphia, PA 19104, USA
(4)
Department of Surgery, University of Pennsylvania, Philadelphia, PA, USA
(5)
Divisions of Cardiovascular Surgery, Penn Presbyterian Medical Center, Philadelphia Heart Institute, Philadelphia, PA, USA
Abstract
Mitral valve repair (MVP) enables preservation of the entire native valve apparatus. Compared to a prosthetic replacement, repairs have been shown to improve postoperative left ventricular function and lower the risk of thromboembolic and bleeding events. Many studies have demonstrated a long-term survival benefit of MVP versus mitral valve replacement. Minimally invasive mitral valve repairs have been shown to be equal in quality and durability, compared to the open approach. Moreover, less invasive methods provide reduced blood loss, fewer transfusions; shorter mechanical ventilator times, and less intensive unit care stays. Also, patients benefit from less pain, better cosmesis, and a shorter overall recovery time. These benefits outweigh the moderate increase in risk afforded by longer cardiopulmonary perfusion and cardiac arrest times. It is probable that in the future, the sternotomy incision will be a used much less in primary mitral valve surgery. Nevertheless, mastering minimally invasive techniques requires a steep learning curve for both the surgeon and operating room staff. This chapter details the use of endoscopic assistance and endoballoon aortic occlusion for minimally invasive mitral valve repairs. These methods should be used in team and surgeon preparation to advance toward totally endoscopic robotic mitral valve surgery. We believe that new technology should be added serially to a mastered operation rather than relying on several complex advancements at once.
Keywords
MitralRepairEndo-balloonAortaEndoscopicRoboticBackground
Current trends indicate that mitral valve repair (MVP) has been underutilized in the past but is becoming more widespread [1, 2]. Indications for mitral valve operative intervention and a repair are detailed in both the current (2008) ACC/AHA and (2012) ESC/EACTS Guidelines [3, 4]. The benefits of MVP include the preservation of the native valve apparatus, improving postoperative left ventricular function, and a lower risk of thromboembolic events. Moreover, the risks of either short or long-term anticoagulation or prosthetic valve failure are obviated. Numerous studies have demonstrated the improved durability and long-term survival benefit of MVP over mitral valve replacement (MVR) [5–7].
Traditionally, mitral valve repair has been approached through a median sternotomy. In the past 15 years, minimally invasive platforms have been developed to access the mitral valve through a right mini-thoracotomy. In 2003, Casselman et al. published their results demonstrating that MVP could be done safely and effectively using a minimally invasive endoscopic approach [8]. Thereafter, endoscopic MVP expanded because of decreased surgical trauma, shorter recovery periods, improved cosmesis, and increased patient satisfaction. Until recently there has been a paucity of literature comparing long-term outcomes to the gold standard open approach. Multiple groups have shown that despite a trend toward increased cardiopulmonary bypass and cross-clamp times, endoscopic MVP can render equivalent short- and long-term survival, valve durability, and freedom from reoperation, when compared to standard repair approaches [9–12].
A right mini-thoracotomy has become the standard incision used for most minimally invasive mitral valve operations. Several techniques have been used to provide safe and adequate exposure to the mitral valve. Trans-thoracic aortic clamping and endo-balloon aortic occlusion both with antegrade/retrograde cardioplegia, as well as hypothermic fibrillatory arrest, all have become acceptable strategies for minimally invasive mitral surgery [11, 12]. Moreover, each of these techniques can be used with advanced robotic approaches to mitral valve repair. Nevertheless, most surgeons prefer to master operating through a mini-thoracotomy using these perfusion and myocardial protection strategies before advancing to using robotic surgical devices. To this end, this chapter will focus on the use of endoscopic assistance and endo-balloon aortic occlusion for minimally invasive mitral valve repairs. We have found that mastery of our method can lessen the learning curve when a surgeon advances to the totally robotic method.
Anesthesia and Operative Preparation
Our patients that undergo a minimally invasive mitral valve operation receive standard cardiac anesthesia and induction. To enable single left lung ventilation with right lung deflation, intubation is done using a double lumen endotracheal tube (Fig. 20.1). The patient is positioned supine on the operating room table with the right hemi-thorax elevated by a shoulder roll or inflatable cushion, and the thighs are abducted slightly to allow access to the femoral vessels. The left arm is tucked, and the right arm is placed along the side in a relaxed anatomic position.
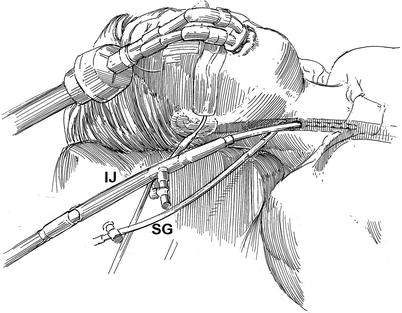

Fig. 20.1
Superior vena and coronary sinus cannulation: the patient is intubated with a double lumen endotracheal tube for right lung isolation. The right internal jugular vein (IJ) is cannulated for venous drainage, using the guide-wire Seldinger technique, and is passed into the superior vena cava under echocardiographic guidance. Using the “double stick” method the either a Swan-Ganz (SG) pulmonary artery or retrograde coronary sinus cardioplegia catheter is passed through the right internal jugular vein and into the superior vena cave and the right atrium. The latter is flow directed onto the pulmonary artery, and the retrograde cardioplegia catheter is positioned in the mid coronary sinus under either echocardiographic or fluoroscopic guidance
A left radial arterial catheter is placed to measure continuous systemic blood pressure In addition, a right radial arterial catheter should be placed to monitor the possibility of Endoclamp™ aortic balloon (Edwards Lifesciences, Inc., Irvine, CA) migration cephalad, which can cause obstruction of the innominate artery origin. Thereafter, using the Seldinger guide-wire method, the right internal jugular vein is cannulated with a 14 or 17 Fr Medtronic™ DLP cannula (Medtronic, Inc., St. Paul MN), which is positioned in the distal superior vena cava for venous drainage (Fig. 20.1). If a retrograde coronary sinus cardioplegia catheter is to be used, it is placed in the right internal jugular vein by the “double-stick” method. A trans-esophageal echocardiographic (TEE) probe is used to position all vascular catheters and access valve/ventricular function. Next, either an Endoboy™ (ASFS Medics, Inc., Noirt, France) or Olympus™ endoscope holder (Olympus Imaging America Inc., Center Valley, PA) is attached to the right side table rail near the patient’s head. The stabilizing post of a Bookwalter-Codman retractor (Symmetry Medical, Inc. Warsaw, IN) is placed near the mid-thorax on the left side of the table to attach the trans-thoracic left atrial retractor (Fig. 20.2).
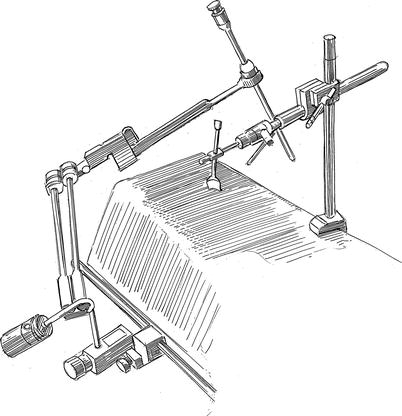

Fig. 20.2
Endoscopic camera holder and left atrial retractor support: either an Endoboy™ or Olympus™ endoscope holder is attached to the right side table rail near the patient’s head. Both devices can secure the 5-mm endoscope in various intra-thoracic and cardiac positions, obviating the need for an assistant to hold the camera. The stabilizing post of a Bookwalter-Codman retractor is placed near the mid-thorax on the left side of the table to attach the trans-thoracic left atrial retractor (see Fig. 20.3)
Operative Technique
A 4.5-cm transverse mini-thoracotomy is made in right fourth intercostal (ICS) space between the mid-clavicular and anterior axillary line (Fig. 20.3). In women the skin incision is placed always in the infra-mammary fold. Before entering the right pleural cavity, the right lung is isolated and collapsed. A 7.5-mm port is placed along the anterior axillary line in the 6th or 7th ICS for carbon dioxide (CO2) insuflation and introduction of a left atrial sump catheter. Continuous, CO2 insufflation 2 l/min helps to displace intra-cardiac air. A 5-mm camera port is placed in the right 4th ICS along the anterior axillary line. Thereafter, an adhesive Edwards Perivue™ soft tissue retractor (Edwards Lifesciences, Inc. Irvine, CA) is inserted through the incision and secured to the chest wall. Under direct vision a 2-mm parasternal incision is made in the fourth interspace for introduction of the trans-thoracic left atrial retractor arm.
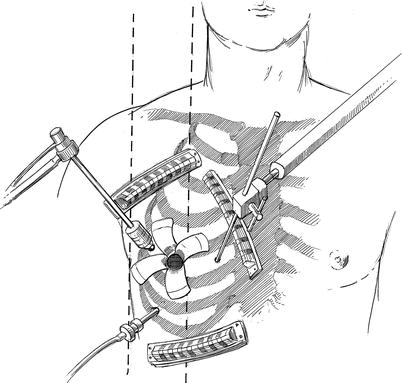

Fig. 20.3




Incision and port placement: a 4.5-cm transverse mini-thoracotomy has been made in right fourth intercostal space between the mid-clavicular and anterior axillary line. The 7.5-mm port, placed along the anterior axillary line in the 6th or 7th ICS, is for carbon dioxide (CO2) insuflation and left atrial suction catheter introduction. A 5-mm camera and port have been through the right 4th ICS along the anterior axillary line. An adhesive Perivue™ (Edwards Lifesciences, Inc., Irvine, Calif.) soft tissue retractor has been inserted through the incision and secured to the chest wall. The trans-thoracic left atrial retractor arm has been inserted through the fourth interspace and the attached to the Bookwalter retractor arm stabilizer (see Fig. 20.2). Adhesive guides help keep individual sutures organized
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


