Fig. 42.1
Airway fistula developing as a result of treatment for lymphoma. (a) A 48-year-old man with B cell lymphoma who underwent chemotherapy and radiation. Lymphoma had eroded into the trachea, and after effective chemotherapy, large airway defect (arrow) was present. (b) Corresponding endoscopic view of tracheal wall defect
Table 42.1
Etiologies of bronchopleural fistula
Postoperatively after lung resection |
Necrotizing pulmonary infection |
Haemophilus influenzae |
Streptococcus viridans |
Staphylococcus aureus |
Pseudomonas aeruginosa |
Klebsiella pneumoniae |
Pneumococcus |
Nonhemolytic streptococcus |
Aspergillus |
Histoplasma capsulatum |
Pulmonary abscess rupture |
Malignancy (lung, thyroid, lymphoma, esophageal) |
Advanced disease with tumor eroding into airway |
Recurrence at stump from prior resection |
Radiation therapy |
Penetrating thoracic trauma |
Complication of percutaneous lung needle biopsy, thoracentesis |
Persistent spontaneous pneumothorax |
Rupture of bullae or cyst |
ARDS |
Gastroesophageal reflux disease with Barrett’s esophagus |
Boerhaave’s syndrome |
Idiopathic |
Postoperative Bronchopleural Fistula
Surgery on the thorax, especially pulmonary resection, is the most common etiology of a bronchopleural fistula (BPF). The incidence is highly dependent on surgical technique, complexity of surgery, and experience of the surgeon. Postoperative bronchopleural fistula has been reported to occur in 1.5–28% of all pulmonary resections. Multiple surgical and nonsurgical risk factors have been associated with the development of postoperative BPF (Table 42.2). Surgical complexity and extensive dissection are important risk factors. Although postoperative BPF may be seen in up to 20% of pneumonectomies, this complication is seen in only about 0.5% of lobectomies. Right-sided surgery is an important risk factor for BPF formation. A 10-year review of surgical data demonstrated almost threefold higher risk of BPF after right pneumonectomy compared to left (13.2% vs. 5.0%, p = 0.047). A subsequent meta-analysis demonstrated BPF to be an independent risk factor for death after right pneumonectomy with a relative risk (RR) of 3.39 for death after right pneumonectomy. Right-sided operations are technically more complicated and more likely to involve extended dissection, hand-sewn closures, closed buttress, or intrapericardial dissection. Postoperatively, right-sided stumps also tend to pool secretions more which can impair complete healing. In patients undergoing pulmonary resection for malignancy, a longer bronchial stump was an independent risk factor for BPF. Patient factors including diabetes mellitus, concurrent steroid use, hypoalbuminemia, cirrhosis, Haemophilus influenzae in sputum, residual tumor at stump, and postoperative mechanical vent for >24-h postsurgery have all been implicated in the development of a postoperative BPF.
Table 42.2
Risk factors associated with increased risk of postoperative bronchopleural fistula
Surgical factors |
Right-sided pulmonary resections (especially right pneumonectomy) |
Excessive peribronchial or paratracheal dissection |
Long bronchial stump/short distance from tumor to stump |
Mediastinal lymph node dissection |
High-dose preoperative radiation therapy |
Residual/recurrent carcinoma at surgical stump |
Postoperative infection (pneumonia, abscess, empyema) |
Nonsurgical factors |
Hypoalbuminemia |
Cirrhosis |
Haemophilus influenzae in sputum |
Residual tumor at stump |
Postoperative mechanical ventilation for >24 h |
Clinical Presentation
Postoperative Patient
The presentation of a bronchopleural fistula developing acutely (within hours to days) after surgery is fairly dramatic. It is heralded as the sudden onset of dyspnea, subcutaneous emphysema, cough w/ purulent sputum, or a life-threatening tension pneumothorax. Thoracic surgery patients invariably have a chest tube in the immediate postoperative period to avoid or immediately identify this complication. A continuous airleak or increase in the output of air in the waterseal chamber of a pleural fluid collection container should alert the physician to the possibility of a bronchopleural fistula. Bronchopleural fistulas identified within the first 4 days postoperative should return for re-exploration and closure of the stump leak if clinical situation allows.
The subacute (postoperative day 7–30) or chronic presentation (postoperative day >30) for a bronchopleural fistula is less impressive. Patients complain of fatigue, wasting, dyspnea, low-grade fevers, or productive cough. Hemoptysis or metalloptysis (coughing of surgical material) has been described. After pneumonectomy, there is an expected degree of and air and fluid present for at least a few weeks. The space is usually obliterated within 7 months. A major decrease in pleural effusion or dramatic change in the air–fluid pattern (increasing pneumothorax, changes in hydropneumothorax level, new air–fluid level) after pulmonary resection should raise concern for a postoperative BPF. Depending on type of surgery and loculation(s) in the pleural space, subtle changes may not be readily visualized on a plain chest radiograph. In the chronic setting, bronchopleural fistulas typically occur as a result of chronic pleural space infection or fibrosis, usually in an immunocompromised patient. Reappearance of air in a previous obliterated space is an ominous sign for BPF. Overall, bronchopleural fistulas are most commonly diagnosed between 8 and 12 days postoperative.
Non-postoperative Patient
The presentation of a BPF in the non-postoperative patient depends on the characteristics of the underlying disease. Most patients will have fever, persistent cough, thick and/or copious sputum production, and a pleural effusion with an air–fluid level. A patient with existing pneumonia or empyema, however, may already be exhibiting these symptoms leading to a delay in diagnosis. A non-resolving pneumonia, infiltrate, or effusion, especially in a patient with underlying lung disease, should warrant further investigation. In patients on mechanical ventilation, an abrupt and significant decrease in airway pressures should raise concerns for BPF formation. Hemoptysis may occur in malignancy-related fistulas, and cough and SOB during eating can be seen with esophageal to airway fistulas.
Diagnosis
Most patients with symptoms compatible with a BPF will initially be evaluated with a chest radiograph. Findings on radiographs may be nonspecific and include pneumothorax, subcutaneous emphysema, and/or pneumomediastinum. In a postoperative patient, a fluid collection adjacent to the stump may be identified. Although a computer tomography (CT) of the chest is much more sensitive at identifying abnormalities related to a BPF, it likely will not identify the location of the fistula itself. In a small study by Westcott and Volpe in 1995, in patients with clinical suspicion for BPF, the fistula site could be isolated on a CT scans in only 50% of the patients. Figure 42.2 demonstrates the radiographic appearance of a BPF.
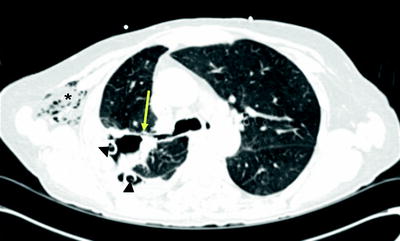

Fig. 42.2
CT appearance of BPF (arrow) in right upper lobe. Note chest tubes (arrowhead) and subcutaneous emphysema (asterisk) in this postoperative patient
Bronchoscopy should be performed in all patients suspected of having a BPF. In large or central lesions, it may be possible to directly visualize the fistula opening. In the case of a suspected postoperative BPF, the stump should be closely examined. If stump dehiscence is not seen, saline should be instilled onto the stump. The presence of continuous bubbling of saline from the stump indicates a fistula is present (Fig. 42.3). In the immediate postoperative patient, installation of methylene blue onto stump can be performed. Its presence in the chest tube output indicates a BPF is present.
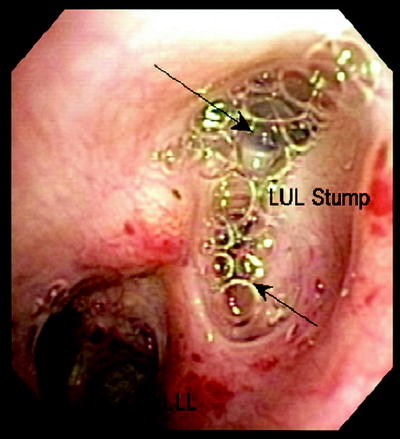

Fig. 42.3
A 70-year-old woman s/p LUL lobectomy with stump leak. Saline injected via flexible bronchoscope indicated continuous air bubbling (arrows) back through stump
If a fistula cannot be seen within the central airways, a bronchoscopy can still be helpful in determining the approximate location of a peripheral bronchopleural fistula. In patients with a chest tube, a balloon can be inserted via the working channel of the bronchoscope and inserted into the segment or subsegment of the airway with suspected fistula (Fig. 42.4). Once inflated, the balloon will occlude airflow through the fistula, and therefore, the airleak will decrease or disappear in the waterseal chamber of a pleural collection device. If the segment does not contain a fistula, inflation of the balloon will have no impact on the airleak. If even the general location of the fistula is unknown, a larger balloon can be inflated in the larger airways before proceeding to the segmental bronchi to provide the bronchoscopist with the general location of the fistula. In some cases, more than one airway may be contributing to a fistula so multiple balloon inflations may be necessary to identify the culprit airways.
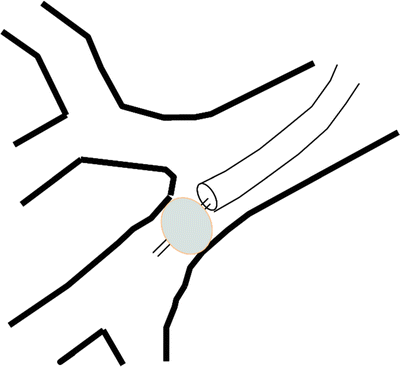

Fig. 42.4
Bronchoscopic localization of a peripheral BPF. Inflating a balloon within airway leading to a BPF will stop airflow into effected airway. This will either (1) slow or stop the airleak in waterseal chamber of pleural collection system or (2) lead to a persistent negative pressure measured by the Chartis™ system
A peripheral BPF can also be identified by a change in the pressure of the airway leading to the fistula. The Chartis System (Pulmonx, Redwood City, CA) is able to measure airflow and pressure in the airway distal to the bronchoscope (Fig. 42.5). It was originally designed to quantify collateral ventilation for endoscopic lung volume reduction. Once the bronchoscope is navigated to the target airway, a balloon catheter is inflated and the airway is occluded. The tip of the catheter has a pressure sensor which provides measurement of airflow and pressure in the occluded airway (Please see Fig. 42.5 for details). As long as the airway is intact and there is adequate seal with the balloon, a small or no drop in pressure will occur with balloon inflation. If the pressure remains negative during both inspiration and expiration, a BPF is present. Capnography (measurement of exhaled carbon dioxide) can also be helpful in identifying the location of a fistula. A polyurethane catheter attached to a capnometer can be placed through the bronchoscope and inserted into sequential airways. During exhalation, carbon dioxide should be detected within the airway. The absence of an end tidal CO2 tracing in a particular segment identifies the segment with the fistula as the carbon dioxide leaks out into the pleural space. Both airway pressure measurement and capnography are useful in identifying BPFs in patients without chest tubes or in situations where subtle changes in bubbling through the waterseal chamber occur when a balloon is occluding airflow.
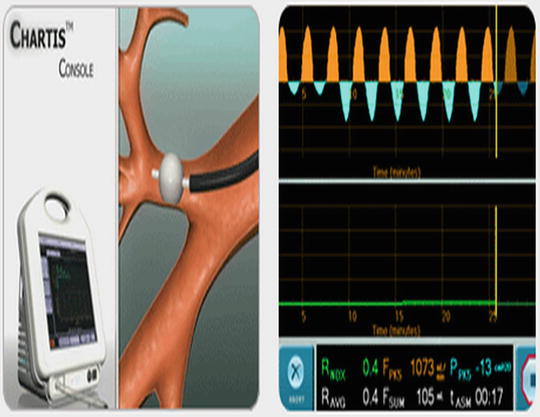

Fig. 42.5
Chartis™ system developed for endoscopic LVRS can be helpful in localizing a BPF. The balloon is inflated and a pressure sensor at the end of the balloon measures both pressure and flow distal to the occluded bronchus. A significant negative pressure during both inspiration and expiration indicates a segment involved in a BPF (© 2010 Pulmonox. All Rights Reserved)
Advanced imaging techniques may be helpful in the diagnosis of a bronchopleural fistula. Bronchography can be done if any of the above-mentioned bronchoscopic techniques are inconclusive. In bronchography, 20–30 mL of a water-based nonionic low osmolar iodinated contrast medium (i.e., Omnipaque, GE Healthcare) is injected through a catheter placed through the working channel of a bronchoscope (Fig. 42.6). Fluoroscopy or CT can then be performed with visualization of contrast media extravasation from a site of a bronchopleural fistula. Scintigraphy with 99mTc-albumin (Technetium-albumin) colloid fog inhalation has been described as a simple and accurate test for the detection of BPF. This is accomplished by aerosolization of a radiotracer and inhalation into the lungs with accumulation of the radiotracer at location of a BPF. This technique requires substantial time and cooperation on the part of a non-intubated patient and can be inconclusive in the setting of small fistulas or underlying lung disease such as COPD. Alternatively, ventilation scintigraphy with other radioactive tracers such as 81mKr (Krypton), 133Xe (Xenon), 99mTc-DTPA (technetium-labeled diethylenetriamine penta-acetate), and 99mTc-sulfur colloid has also been described. All of the advanced radiographic techniques mentioned above are now infrequently used with the advent and safety of balloon occlusion of the airway. Historically, bronchography was used for many years with excellent diagnostic accuracy.
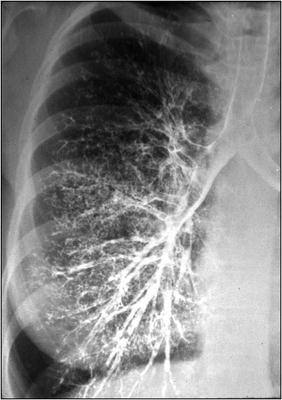

Fig. 42.6
Normal bronchogram. Contrast material is injected into the airway via a bronchoscope and fluoroscopy is performed. Extravasation of contrast material in the pleural space indicates the presence of a bronchopleural fistula (Image courtesy of Stefan Tigges, M.D.)
Prognosis
In general, there is a high morbidity and mortality associated with bronchopleural fistulas. Mortality generally falls in the range of 20–70%, depending on the underlying disease process. Postoperative fistulas which can be surgically repaired have a lower morbidity and mortality than fistulas related to underlying malignancy or infection. Even with successful intervention, mortality still can be as high as 40%. Death is usually related to a combination of aspiration and recurrent infections (pneumonia, empyema) which may lead to the development of acute respiratory distress syndrome and multiorgan system failure.
Treatment
General Principles
The initial management of any BPF should first address any immediate, life-threatening conditions, such as pleural space contamination, pulmonary flooding, or tension pneumothorax. A chest tube, if not already in place, should be inserted immediately to address these concerns. In complex cases with pleural adhesions and/or loculated hydropneumothoracies, multiple well-positioned and/or image-guided chest tubes may be required. In the case of BPF due to a necrotizing lung or pleural space infection, a trial of antibiotics with adequate drainage should be attempted to decompress the pleural space, allowing time for full lung re-expansion and healing of the fistula. With severe necrotizing pneumonia, many weeks of antibiotic therapy, nutritional supplementation, patient rehabilitation, and chronic pleural space drainage may be required before contemplating surgery. Although it is tempting to surgically close a fistula, active infection in the lung parenchyma or pleural space can lead to worse outcomes in the acute setting.
Conservative Therapy
Bronchopleural fistulas are very difficult to manage while the patient is on mechanical ventilation. Ventilator-delivered breaths will preferentially flow through the fistula as it represents the lowest point of resistance in the airway. This leads to difficulties with oxygenation and loss of exhaled tidal volumes and subsequent hypercapnia. Limiting airway pressure is an important strategy as continued airflow through the tract delays natural healing. Minimization of positive end expiratory pressure (PEEP), inspiratory flow rate, and tidal volume should be attempted to the extent tolerated by the patient. In large fistulas, selective intubation of the contralateral lung may be necessary to completely cease any airflow through the fistula. High frequency oscillatory ventilation has been studied and found to be slightly more beneficial in patients with a proximal BPF and lack of parenchymal disease.
After initial drainage of the pneumothorax, the chest tube may actually contribute to the persistence of the BPF. In patients with minimal or no residual pneumothorax, suction should be removed and the chest tube placed on waterseal. Keeping the chest tube on suction may have a paradoxical effect by “pulling” the defect open and contributing to the persistence of the BPF. Withdrawal of suction from the chest tubes minimizes airflow through the fistula to allow improved healing of the tract. Installation of a pleural sclerosing agent (talc, bleomycin, etc.) through the chest tube into the pleural space is often attempted as a minimally invasive method for sealing an airleak. The goal is to fuse (pleurodese) the visceral and parietal pleura together, which will either contain the airleak or incite an inflammatory response to close the fistula. In order for this to be successful, the lung needs to completely fill the hemithorax so that there is good apposition between the visceral and parietal pleural surfaces. If a large pneumothorax is present, pleurodesis will not be achieved and the airleak will persist.
Fistula Closure
Conservative measures (as described above) for closure of a bronchopleural fistula are necessary in patients who are poor surgical candidates or have small peripheral defects which do not necessitate more aggressive intervention. In cases where conservative treatment fails, localization of the fistula is paramount in successful closure of the fistula either by semi-invasive (bronchoscopic) or invasive (surgical) management. There have been no large studies describing the optimal treatment or outcomes for bronchopleural fistulas nor does consensus opinion exist to suggest an optimal treatment in any particular situation. Regardless of the cause of the fistula, endoscopic and surgical treatment should not be viewed in isolation; instead, they can be complementary techniques.
Surgical Closure of BPF
If a postoperative BPF is detected within a few days of the patient’s original surgery, re-exploration by either VATS or open thoracotomy with closure of the fistula is the recommended approach if the clinical situation allows. The success rate of BPF closure with surgery has been reported to be as high as 80–95%, although this includes the postoperative population that is healthy enough to undergo a major reoperation. There are multiple surgical options described for closure of a BPF: (1) VATS/thoracotomy with direct resection and closure of the stump with intercostal muscle reinforcement or omental flap, (2) trans-sternal bronchial closure, (3) thoracoplasty with or without extrathoracic chest wall muscle transposition, or (4) chronic drainage with chest tube or Eloesser procedure (described below). Sequential operative procedures are often planned in debilitated patients who would not tolerate another major operation. Bronchopleural fistula complicated by empyema is one such circumstance where sequential operations may achieve better success. The pleural cavity is allowed to continuously drain by an empyema tube or an Eloesser procedure. The Eloesser procedure was named after Leo Eloesser, the thoracic surgeon who first described the technique. It involves the creation of a 5-cm opening in the chest wall with resection of 2–3 adjacent ribs and suturing of the skin to the pleural cavity to allow complete continuous drainage of the pleural space. The Eloesser procedure has minimal morbidity even in chronically ill patients and can be used in the acute setting. The patient then undergoes aggressive nutritional support and intensive physical rehabilitation and returns to the operating room once healthy enough to tolerate a second procedure for a thoracotomy with flap closure of the BPF. The details and technical aspects of surgical closure of bronchopleural fistulas are beyond the scope of this discussion. (Please refer to suggested reading at the end of this chapter for additional resources.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


