Fig. 8.1
The effect of thyroid hormones on the heart
Amiodarone, a benzofuranic iodine-rich antiarrhythmic drug, causes thyroid dysfunction in 15–20% of treated patients, including both hypothyroidism and thyrotoxicosis. Amiodarone-induced hypothyroidism results from persistent iodine-induced inhibition of thyroid gland function and is more prevalent in patients with preexisting thyroid autoimmunity. Amiodarone could also induce thyrotoxicosis of two forms: iodine-induced hyperthyroidism or destructive thyroiditis. Type 1 results in the synthesis and release of excess thyroid hormone, whereas type 2 results in the release of preformed thyroid hormone from the inflamed thyroid gland [13].
8.3 Parathyroid and the Cardiovascular System
Parathyroid hormone regulates the body’s calcium levels. Hypercalcemia induces cardiac effects including syncope from arrhythmias, whereas hypoglycemia may result in refractory hypotension or arrhythmias. Calcium plays a key role in heart muscle contraction and relaxation. Hypocalcemic heart failure is a rare and potentially reversible disturbance, which reflects its intrinsic relationship. In this clinical condition, the ECG shows severe global left ventricular dysfunction, normal coronary arteries at cardiac catheterization, severe hypocalcemia, and new-onset hypoparathyroidism. This condition completely normalizes after metabolic stabilization considering hypocalcemia as a cause of reversible myocardial dysfunction. Hypercalcemia, acute and chronic, is known to have effects on the heart and the vascular system that are potentially life-threatening. Some of these effects include accelerated atherosclerosis, uncontrolled hypertension, structural effects, and progressive cardiac dysfunction. Figure 8.2 shows the ECG modifications induced by hypocalcemia and hypercalcemia. Hypocalcemia causes a long QT, whereas hypercalcemia induces a shortening of the QT interval.
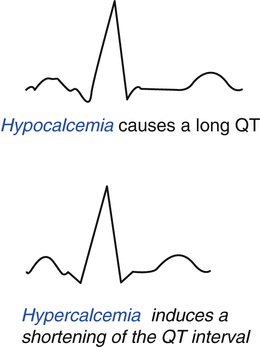

Fig. 8.2
Electrocardiographic alterations induced by hypocalcemia and hypercalcemia
8.4 Adrenal Gland and the Cardiovascular System
Of all the hormones secreted by the adrenal glands, those involved in cardiovascular regulation are glucocorticoids, mineralocorticoids, and adrenomedullary hormones. Glucocorticoids released by the adrenal cortex include hydrocortisone, commonly known as cortisol, which regulates mechanisms inducing the conversion of fats, proteins, and carbohydrates to energy. It also helps in the regulation of blood pressure and cardiovascular function. The principal mineral corticoid is aldosterone, which maintains the balance of salt and water and helps to control blood pressure. The hormones of the adrenal medulla are released after the sympathetic nervous system is stimulated, which occurs during stress, and stimulate the heart rate. Epinephrine, well-known as adrenaline rapidly responds to stress by increasing the heart rate and rushing blood to the muscles and brain. Norepinephrine works with epinephrine in response to stress, causing vasoconstriction, increasing blood pressure. Hypercortisolism is associated with hypertension, central obesity, insulin resistance, dyslipidemia, and alterations in the clotting and platelet functions. Patients with hypercortisolism may have impaired fasting glucose, impaired glucose tolerance, hyperinsulinemia, insulin resistance, and/or diabetes mellitus. Cushing’s syndrome has been associated with increased lipoprotein(a), decreased HDLc, and increased triglycerides. The duration of cortisol excess correlates with the degree of dyslipidemia seen. Cortisol also increases the synthesis of several coagulation factors, stimulating endothelial production of von Willebrand factor and concomitantly increasing factor VIII. Hypercortisolism may also enhance platelet aggregation and reduce plasma fibrinolytic capacity [4]. Moreover, hyperaldosteronism causes maladaptive cardiac remodeling and has been associated with heart failure, cardiac fibrosis, and diastolic dysfunction [14]. Aldosterone has also been shown to promote collagen deposition, activate inflammatory cells, and stimulate fibroblast proliferation [15]. Surgical and medical treatments may be effective in reducing left ventricular mass, with decreases in blood pressure and plasma aldosterone levels predictive of response to therapy. At the very least, excess catecholamine action in pheochromocytoma can lead to cardiomyopathy, ischemic heart disease, myocardial stunning, and cardiogenic shock. Echocardiogram may reveal left ventricular dilatation with a diffuse decrease in contractility, left atrial dilatation with increased end-diastolic pressure, reduced ejection fraction, and septal hypertrophy. In the setting of intravascular volume depletion and impaired diastolic filling, patients may present with an outflow obstruction that mimics hypertrophic obstructive cardiomyopathy [16].
8.5 Diabetes Mellitus and the Cardiovascular System
In patients affected by diabetes, atherosclerotic cardiovascular disease is the leading cause of morbidity and mortality and represents the major determinant of direct and indirect costs related to the disease. Diabetes is associated with a two- to four-fold increased mortality risk for heart disease together, with significantly higher mortality after myocardial infarction and a worse overall prognosis in coronary heart disease [17]. The common conditions coexisting with diabetes (e.g., hypertension and dyslipidemia) are clear major risk factors for atherosclerotic cardiovascular disease, and the chronic hyperglycemia itself confers a further independent risk [18]. However, the contribution of glucose-lowering to the reduction of macrovascular complications appears to be controversial.
In type 1 diabetes, follow-up results from a large randomized clinical trial suggest that the improvement of metabolic control, obtained through intensive insulin treatment, might prevent cardiovascular events in the long term [19]. On the other hand, despite some encouraging results, the large clinical trials designed to test the efficacy of tight glycemic control in patients affected by type 2 diabetes, with near-normal glycemic control for a median of 3.5–5 years failed to show a significant reduction of cardiovascular events within that period [20]. The increase in iatrogenic hypoglycemia incidence strictly related to antidiabetic therapies at high hypoglycemic risk (e.g., sulfonylureas and insulin) may represent a plausible explanation by determining adrenergic activation, oxidative stress, and cardiac repolarization, leading to cardiac ischemia or fatal arrhythmia [21].
Moreover, concerns have been raised regarding the fact that different glucose-lowering drugs, irrespective of their action on glycemic control, may exert different effects on the cardiovascular risk profile. Although available clinical data ruled out any overall harmful cardiovascular effect of metformin unless when it is added to sulfonylureas, sulfonylureas themselves, insulin, and thiazolidinediones have been suspected of negative cardiovascular effects, although some results were not confirmed by subsequent investigations [22]. To date, the Food and Drug Administration requires preapproval and post-approval studies for all new antidiabetic drugs to rule out excess cardiovascular risk. Despite growing in vivo and in vitro evidence for the cardiovascular benefits, only neutral effects on cardiovascular risk have been demonstrated for incretin-based therapy (e.g., GLP-1 receptor agonist and dipeptidyl peptidase, DPP-44 inhibitors) through large clinical trials with cardiovascular endpoints [23, 24]. The diverging results regarding the relationship between DPP-4 inhibitors and heart failure require further investigation.
More recent data coming from the EMPA-REG Outcome study [25] showed that therapy with sodium-glucose cotransporter 2 inhibitors, the newest antidiabetic drug class, significantly reduced cardiovascular risk in type 2 diabetic patients at high cardiovascular risk. Empagliflozin, then, is the first antidiabetic drug associated with cardiovascular positive effects, with a 38% reduction in cardiovascular death. Further studies to investigate whether this treatment will have similar effects in lower-risk diabetic patients are required.
Recommendations for cardiovascular disease and risk management from the American Diabetes Association pointed out that large benefits are seen only when multiple risk factors are addressed simultaneously. As a consequence, diabetes care implies a multifactorial management of cardiovascular risk, which includes multiple therapeutic goals beyond glycemic control.
8.6 Obesity and the Cardiovascular System
Obesity has been shown to have several negative effects through thrombogenic, atherogenic, oncogenic, hemodynamic, and neurohumoral pathways and has been linked to several chronic diseases, such as diabetes, hypertension, dyslipidemia, and cardiovascular disease, together with malignancies. Overweight and obesity constitute the fifth leading risk for global death according to the World Health Organization. At least, 2.8 million subjects die each year for complications related to overweight/obesity. Moreover, the 44% of diabetes and 23% of ischemic heart disease burden can be attributed to an excess of adipose tissue [26].
Obesity, according to the World Health Organization, is defined by a body fat representation >25% in men and >35% in women at bioelectrical impedance analysis. In clinical practice, overweight and obesity are traditionally diagnosed if body mass index (BMI) is ≥26 and ≥30 kg/m2 respectively. However, several studies have shown a paradoxical relationship between BMI and all-cause and/or cardiovascular mortality, identifying a higher survival rate in overweight in comparison with normal weight or obesity [27–31].
Thus, obesity cannot be adequately ruled out by the BMI calculation, which showed a low sensitivity, missing more than 50% of people with excessive fat mass [32]. Further studies demonstrated a significant association between visceral adiposity, better than BMI, mortality, and cardiovascular disease. Waist circumference as a surrogate measure of abdominal adipose tissue has been placed as one of the main contributors to the metabolic syndrome by the National Cholesterol Education Program Adult Treatment Panel III in 2001 [33] and then as the core feature of the diagnostic criteria proposed by the International Diabetes Federation in 2005.
A possible mechanism linking obesity with cardiovascular disease is subclinical low-grade inflammation. The adipose tissue is recognized as an endocrine organ, capable of synthesizing a large number of biologically active cytokines that regulate metabolic homeostasis. Obesity, and in particular, excess visceral adiposity, results in many qualitative changes in the cellular composition of the tissue itself, including alterations in the number and phenotype of the adipocytes, infiltration by immune, vascular, and structural cells, thus determining a relevant dysregulation of the secretion of those cytokines referred to as adipokines. The overexpression of the pro-inflammatory adipokines (leptin, TNF, IL-6, resistin, retinol-binding protein 4 [RbP4], lipocalin 2, IL-18, angiopoietin-like protein 2 [ANGPTL2], CC-chemokine ligand 2 [CCL2], CXC-chemokine ligand 5 [CXCL5], and nicotinamide phosphoribosyltransferase [NAmPT]), together with impairment of the anti-inflammatory ones (adiponectin and secreted frizzled-related protein 5 [SFRP5]), leads to the development of a chronic inflammatory state [34]. This condition contributes to metabolic dysfunction, which has a deleterious effect on the cardiovascular system, leading to the development of endothelial dysfunction, myocardial ischemia, and cardiomyopathy.
Moreover, recent data have pointed to the important role of a multi-organ cross talk also involving cytokines and other peptides secreted from skeletal muscles in response to exercise with local effects within the muscle or by targeting distant organs. Such proteins are recognized as myokines (e.g., IL-6 and irisin) and represent important contributors of the beneficial metabolic effects of exercise [35]. Skeletal muscle plays a critical role in the glucose metabolism and peripheral insulin sensitivity, and its impairment is commonly related to the increase in adipose tissue, leading to a condition defined as sarcopenic obesity. Adipose tissue excess could underlie the development of sarcopenia. A possible role of vitamin D in sarcopenia has been postulated in two studies, demonstrating that serum 25-hydroxy vitamin D was negatively correlated with appendicular (legs and arms) fat mass and positively associated with appendicular muscle mass, both evaluated through DEXA analysis [36].
Thus, the use of traditional anthropometric measures and lifestyle modifications to characterize and adequately manage patients affected by obesity could be misleading.
8.7 Gonads and the Cardiovascular System
8.7.1 Male
In the cardiovascular system, androgens display predominantly genomic effects, but there is also evidence for nongenomic effects. Genomic effects involve the transcription of specific segments of DNA through the binding of testosterone or dihydrotestosterone to the androgen receptor, which is widely expressed in the cardiovascular system, particularly in vascular smooth muscle, endothelial cells, myocardial fibers, and macrophages. The nongenomic effects of androgens are rapid compared with the genomic effects and include the activation of kinase signaling cascades and the modulation of intracellular calcium levels. Moreover, testosterone also exerts indirect effects through the activation of estrogen receptors, after aromatization into estradiol by the aromatase (P450a) enzyme [37].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


