Tako-Tsubo cardiomyopathy (TTC) is characterized by apical ballooning of the left ventricle and symptoms and signs mimicking acute myocardial infarction. The high catecholamine levels in the acute phase of TTC and common emotional triggers suggest a dysregulated stress response system. This study examined whether patients with TTC show exaggerated emotional, neurohormonal, and hemodynamic responses to mental stress. Patients with TTC (n = 18; mean age 68.3 ± 11.7, 78% women) and 2 comparison groups (healthy controls, n = 19; mean age 60.0 ± 7.6, 68% women; chronic heart failure, n = 19; mean age 68.8 ± 10.1, 68% women) performed a structured mental stress task (anger recall and mental arithmetic) and low-grade exercise with repeated assessments of negative emotions, neurohormones (catecholamines: norepinephrine, epinephrine, dopamine, hypothalamic-pituitary-adrenal axis hormones: adrenocorticotropic hormone [ACTH], cortisol), echocardiography, blood pressure, and heart rate. TTC was associated with higher norepinephrine (520.7 ± 125.5 vs 407.9 ± 155.3 pg/ml, p = 0.021) and dopamine (16.2 ± 10.3 vs 10.3 ± 3.9 pg/ml, p = 0.027) levels during mental stress and relatively low emotional arousal (p <0.05) compared with healthy controls. During exercise, norepinephrine (511.3 ± 167.1 vs 394.4 ± 124.3 pg/ml, p = 0.037) and dopamine (17.3 ± 10.0 vs 10.8 ± 4.1 pg/ml, p = 0.017) levels were also significantly higher in patients with TTC compared with healthy controls. In conclusion, catecholamine levels during mental stress and exercise were elevated in TTC compared with healthy controls. No evidence was found for a dysregulated hypothalamic-pituitary-adrenal axis or hemodynamic responses. Patients with TTC showed blunted emotional arousal to mental stress. This study suggests that catecholamine hyper-reactivity and not emotional hyper-reactivity to stress is likely to play a role in myocardial vulnerability in TTC.
Tako-Tsubo cardiomyopathy (TTC) is characterized by apical ballooning of the left ventricle (LV) and symptoms and signs mimicking acute myocardial infarction. Emotional triggers are common in TTC, and the high catecholamine levels on admission suggest a dysregulated stress response system. This study examined emotional, neurohormonal, and hemodynamic responses to acute mental stress, comparing patients with TTC to healthy controls and cardiac patients with heart failure (HF). We investigated whether emotional, neurohormonal (catecholamines and hypothalamic-pituitary-adrenal [HPA]-related measures adrenocorticotropic hormone [ACTH] and cortisol), and hemodynamic (LV function, blood pressure, and heart rate [HR]) responses to a structured mental stress task are exaggerated in patients with a history of TTC compared with healthy controls and patient controls with stable HF. We also examined potential response patterns to low-grade exercise challenge for comparison purposes.
Methods
From January 2012 to April 2014, 56 patients (18 patients with TTC, 19 healthy controls, and 19 HF control patients) participated in the study. Patients with TTC were identified from the echocardiography patient database belonging to the Elisabeth Tweesteden Hospital, Tilburg, The Netherlands. Patients who were admitted with a diagnosis of TTC in the preceding 5 years were approached by a cardiologist for participation in the study. The diagnosis of TTC was based on the Mayo Clinic diagnostic criteria: (1) akinesia or dyskinesia of the apical and/or midventricular segments of the LV with regional wall motion abnormalities that extend beyond the distribution of a single epicardial vessel; (2) signs and symptoms suggesting ACS (i.e., new-onset electrocardiographic abnormalities such as ST-segment elevation and/or T-wave inversion, [modest] elevation in cardiac troponin levels, and/or typical angina complaints); and (3) absence of obstructive coronary artery disease, pheochromocytoma or myocarditis that could account for the condition. The median time between the acute TTC event and the current assessments was 11.5 months (interquartile range 3.0 to 39.5 months, range 2 to 51). A control group of healthy participants was recruited by advertisement. The healthy controls were matched for gender (using frequency-based matching) and required not to have a history of TTC, coronary artery disease, HF, or other cardiovascular diseases. Echocardiography was used to rule out cardiac pathology. A second control group consisting of patients with stable HF (New York Heart Association class I to II) without a history of TCC was recruited from the same hospital as the patients with TTC and were also matched for gender (n = 19). A total of 8 HF control patients had a history of MI and 5 had previous bypass surgery.
Exclusion criteria for participating in the study were (1) age >85 years, (2) current active treatment for cancer or another life-threatening condition, (3) currently on hormone replacement therapy, and (4) cognitive impairment interfering with completion of tasks. The protocol was approved by the Institutional Review Board (#NL35988.008.11). All participants provided informed consent before participating in the study.
After giving informed consent, participants were equipped with a blood pressure monitor. An indwelling catheter was placed for the repeated blood draws, and a baseline echocardiogram was obtained. The participant rested for 30 minutes for baseline measures. Participants then performed a structured mental stress task (anger recall and mental arithmetic), rested again for 30 minutes to obtain full recovery, and performed a low-intensity physical challenge task, and the protocol was completed with a second recovery period of 30 minutes. Emotions, blood samples, and hemodynamic measures were obtained during the different phases of the protocol as described subsequently. Patients remained supine throughout the entire procedure to minimize effects of posture change on catecholamine levels and hemodynamic measures.
The selected stress manipulations have been extensively validated in previous research and followed a standardized protocol carried out by trained personnel. The mental stress task combined an anger recall speech task and a mental arithmetic task as described previously. The anger recall task required participants to report about a recent incident that elicited intense frustration and anger. The mental arithmetic task immediately followed the anger recall and involved subtraction of serial 7s from a 4-digit number, whereas the patient was encouraged to work as fast and accurately as possible for 5 minutes. We and others, e.g., Blumenthal et al and Steptoe et al, have found these stress tasks to be a potent trigger of hemodynamic and neurohormonal responses in patients with coronary artery disease. Previous research has demonstrated that the effects induced by these mental stress tasks are safe in clinical populations with coronary artery disease and that physiological and cardiovascular responses are not confounded by talking. The physical challenge involved a supine bicycle task with minimal resistance for 5 minutes. Participants peddled at 1 cycle per 2 seconds, and the pace was indicated by means of a metronome. Physical exertion levels were validated using the Borg Perceived Exertion Scale.
Emotions were assessed before (i.e., at the end of baseline) and at the end of the mental stress task and at the end of the physical stress task. Participants rated mood adjectives on a 7-point Likert scale as used previously. Emotions were categorized into 3 subscales: “distress” (tense, stressed, anxious, feeling at ease [reverse-scored]), “irritability” (irritated, annoyed, angry), and “arousal” (challenged, interested). The reliability of the emotional items was good with a Cronbach’s alpha of 0.721.
A 18-gauge intravenous catheter was placed in the antecubital vein, and blood samples were obtained at the end of baseline (after 25 minutes rest), 3 minutes into the anger recall task, 25 minutes into the poststress recovery period, and 3 minutes into the physical stress task. The first 5 ml of each blood draw were discarded to avoid artifacts related to dilution. Blood samples were collected in 6-ml K 2 -ethylene-diamine-tetraacetic acid (EDTA) vacutainer tubes (Beckton & Dickenson, Amsterdam, The Netherlands), immediately stored on ice water, and within 30 minutes after collection, centrifuged for 15 minutes at 3,000 g at 4°C. An aliquot (1.0 ml) was transferred into vials containing Na Metabisulfite (10.0 mg), before storage to avoid oxidation-related inaccuracies of catecholamine analysis. Aliquots of plasma were stored at −80°C until analysis.
Epinephrine, norepinephrine, and dopamine were measured in plasma using isotope dilution mass spectrometry at the University Medical Center Groningen. Catecholamine data were available for 15 of 18 patients with TTC, 19 of 19 controls, and 7 of 19 patients with HF. Catecholamines were not assayed for 11 patients with HF because their EDTA samples were not stored with Na Metabisulfite. The detectable range for epinephrine, norepinephrine, and dopamine were 5.5 to 1,704, 0.68 to 5,330, and 3.0 to 1,088 pg/ml, respectively, and the interassay coefficients of variation [CVs] were <7%, <6%, and <8%, respectively.
Measures of ACTH in K 2 -EDTA samples were performed on a Cobas e 601 analyzer (Roche Diagnostics GmbH, Bazel, Switzerland) using an electrochemiluminescence immunoassay (Roche Diagnostics GmbH, Mannheim, Germany). The detection range for this assay is 0.999 to 1,998 pg/ml (normal range 7.2 to 63.3 pg/ml), and the interassay CV ranged from 5.4% to 3.7% for ACTH concentrations of 5.0 to 1,444.0 pg/ml.
Cortisol was assayed from saliva samples. Samples were collected at the end of baseline rest, 20 minutes after mental stress task, and 20 minutes after physical stress. Participants were asked to chew on a cotton swab for 45 to 60 seconds, and then the swab was placed in a special plastic tube (Salivette; Sarstadt, Newton, North Carolina). Salivettes were kept at −20°C until processing. All saliva samples were analyzed using an enzyme-linked immunosorbent assay (IBL, Hamburg, Germany), and samples of the same participant were run on the same plate. The within-assay CV% of the cortisol assay is 2%.
Two-dimensional echocardiography (iE33; Philips, Best, The Netherlands) was used to examine changes in LV wall motion and global LV function. Optimal visualization of the LV was determined before the study with the participant in supine or semi-supine left lateral decubitus position. An echocardiogram was obtained before the study to rule out severe (new) cardiac pathology. All echocardiograms were performed and interpreted by the same experienced physician sonographer to minimize interobserver variability. Ejection fraction (EF) was measured using biplane Simpson method at baseline, 3 minutes into the mental arithmetic task, and 5 minutes into the exercise task. Wall motion abnormalities were assessed by examining 16 LV segments using side-by-side comparisons.
Systolic blood pressure (BP), diastolic BP, and HR were obtained using an automated cuff (Omron Healthcare, Inc., Vernon Hills, Illinois) placed on the nondominant arm throughout all phases of the experiment. Baseline systolic BP, diastolic BP, and HR levels were determined by averaging the measurements at 23, 25, and 27 minutes into rest. Hemodynamic responses to the stress tasks were assessed at 2-minute intervals during the tasks and recovery; average values during anger recall and arithmetic and exercise tasks were used for analyses.
Demographic variables and medical history were obtained using a semi-structured interview and by reviewing medical records. Sociodemographic measures included age, gender, marital status, having children, education level, and work status. Smoking status, alcohol consumption, weight, and height were assessed from self-reported data, and body mass index was calculated as kilogram per square meter. Medical background variables included history of myocardial infarction, percutaneous coronary intervention, coronary artery bypass graft, atrial fibrillation, noncardiac co-morbidities (stroke, chronic obstructive pulmonary disease, cancer, kidney disease or liver disease, and stomach ulcer), and cardiac risk factors (hypertension, hypercholesterolemia, and diabetes mellitus). Medication use was assessed including use of β-adrenergic blocking agents, ACE inhibitors, diuretics, short- and long-acting nitrates, antiplatelet medication, lipid-lowering drugs, antidepressive medications, and benzodiazepines.
Data are presented as mean ± SD or n (%). We used the natural logarithm of the raw catecholamine values for statistical analyses to limit bias related to non-normal distribution of the data. Task responses were examined using paired-sample t tests for within-group differences. Differences between groups were analyzed using independent-samples t tests. Repeated-measures analyses of variance was used to assess emotional, neurohormonal, and hemodynamic responses during the different stages of the experiment using general linear models, with “task” as the repeated within-subject factor and patient group (TTC, HF control patients, and healthy controls) as between-subjects factor, adjusting for age and gender. Repeated-measures analyses were conducted for mental stress and exercise separately. We used IBM SPSS Statistics 22 (IBM Corp., Armonk NY) to perform all analyses, and a 2-sided p value <0.05 was considered statistically significant.
Results
Characteristics of the study sample are listed in Table 1 . Patients with TTC were on average older compared with the healthy control group and, thus, less likely to be used. Age and gender were adjusted for multivariable analyses. As listed in Table 1 , patients with TTC were also more likely to have hypertension and use β-adrenergic blocking agents, ACE inhibitors, antiplatelet medication, and lipid-lowering medication. Patients with TTC did not differ from patients with HF on any of the demographic variables but were less likely to have diabetes mellitus or use diuretics, nitrates, or antiplatelet medication compared with patients with HF. Among the 18 patients with TTC, 10 patients (56%) reported a triggering event related to their TTC. A psychological trigger was reported by 9 patients and only 1 patient reported a physical trigger. No clear triggering event was reported by 7 patients, and 1 patient could not remember the circumstances before hospital admission for TTC.
| Variable | Tako-Tsubo N=18 (32%) | Heart failure (HF) N=19 (34%) | p- value TTC vs. HF | Healthy control (HC) N=19 (34%) | p- value TTC vs. HC |
|---|---|---|---|---|---|
| Female | 14 (78%) | 13 (68%) | 0.522 | 13 (68%) | 0.522 |
| Age (years) | 68.3±11.7 | 68.8±10.1 | 0.888 | 60.0±7.6 | 0.014 |
| Married | 11 (61%) | 14 (78%) | 0.278 | 14 (74%) | 0.414 |
| Children | 15 (83%) | 17 (94%) | 0.289 | 15 (79%) | 0.734 |
| Higher education | 3 (17%) | 2 (12%) | 0.679 | 8 (42%) | 0.091 |
| Employed | 3 (17%) | 3 (17%) | >.999 | 13 (68%) | 0.001 |
| Smoker | 3 (17%) | 5 (28%) | 0.423 | 0 | 0.063 |
| Alcohol user | 11 (61%) | 8 (44%) | 0.317 | 16 (84%) | 0.114 |
| Body mass index (kg/m 2 ) | 26.2±5.1 | 29.6±5.2 | 0.053 | 26.0±3.9 | 0.898 |
| Medical History | |||||
| Atrial Fibrillation | 1 (6%) | 3 (16%) | 0.316 | 0 | 0.298 |
| Hypertension | 15 (83%) | 17 (90%) | 0.585 | 6 (32%) | 0.001 |
| Hypercholesterolemia | 8 (44%) | 12 (63%) | 0.254 | 3 (16%) | 0.057 |
| Diabetes mellitus | 1 (6%) | 9 (47%) | 0.004 | 0 | 0.298 |
| Stroke | 1 (6%) | 1 (6%) | >0.999 | 0 | 0.298 |
| Chronic Obstructive Pulmonary Disease | 1 (6%) | 4 (22%) | 0.148 | 0 | 0.298 |
| Cancer | 4 (22%) | 4 (25%) | 0.849 | 2 (11%) | 0.335 |
| Kidney or Liver disease | 2 (11%) | 1 (6%) | 0.581 | 0 | 0.135 |
| Stomach ulcer | 2 (11%) | 0 | 0.146 | 0 | 0.135 |
| Medications | |||||
| Beta-blockers | 12 (75%) | 14 (88%) | 0.365 | 3 (16%) | <0.001 |
| ACE-inhibitors | 11 (61%) | 15 (79%) | 0.235 | 1 (5%) | <0.001 |
| Diuretics | 4 (22%) | 16 (84%) | <0.001 | 3 (16%) | 0.618 |
| Vasodilators | 1 (6%) | 8 (50%) | 0.006 | 0 | 0.269 |
| Antiplatelet | 13 (77%) | 16 (100%) | 0.038 | 1 (5%) | <0.001 |
| Lipid lowering | 7 (41%) | 10 (63%) | 0.221 | 2 (11%) | 0.034 |
| Anti-depressants | 1 (6%) | 2 (13%) | 0.544 | 0 | 0.269 |
| Benzodiazepine | 2 (13%) | 2 (13%) | >.999 | 0 | 0.112 |
Emotional responses to mental stress are displayed in Table 2 . Significant increases were found in “distress” and “irritability” in response to the anger recall task and the arithmetic task compared with baseline (p <0.001), and “arousal” showed a borderline statistically significant increase (p = 0.076). During both the anger recall and arithmetic task, patients with TTC reported lower levels of arousal compared with healthy controls. No other differences in self-reported emotions were observed between TTC and both the healthy and HF control groups. Baseline emotional states were also not different in patients with TTC versus the control groups.
| Variable | Tako-Tsubo N=18 (32%) | Heart failure N=19 (34%) | p- value TTC vs. HF | Healthy control N=19 (34%) | p- value TTC vs. HC |
|---|---|---|---|---|---|
| Baseline | |||||
| Distress | 1.8 ± 0.8 | 1.7 ± 0.9 | 0.845 | 1.3 ± 0.6 | 0.053 |
| Irritability | 1.3 ± 0.6 | 1.2 ± 0.6 | 0.517 | 1.3 ± 0.8 | 0.760 |
| Arousal | 2.9 ± 1.4 | 3.4 ± 1.1 | 0.258 | 3.2 ± 1.3 | 0.637 |
| Anger recall | |||||
| Distress | 3.4 ± 1.6 | 3.50 ± 1.4 | 0.889 | 4.1 ± 1.4 | 0.182 |
| Irritability | 3.5 ± 2.1 | 3.5 ± 1.9 | 0.992 | 4.3 ± 1.5 | 0.163 |
| Arousal | 3.2 ± 1.5 | 3.5 ± 1.7 | 0.636 | 4.2 ± 1.4 | 0.049 |
| Arithmetic | |||||
| Distress | 4.5 ± 1.6 | 4.1 ± 1.2 | 0.402 | 4.1 ± 1.4 | 0.350 |
| Irritability | 3.7 ± 2.0 | 3.2 ± 1.6 | 0.409 | 3.8 ± 1.9 | 0.896 |
| Arousal | 4.1 ± 1.0 | 4.3 ± 1.4 | 0.471 | 5.0 ± 1.1 | 0.008 |
Repeated-measures analyses of variance of the task-induced emotional responses showed a consistent pattern. For the arousal response, there was a main effect for group (F 2,51 = 4.552, p = 0.015, partial η 2 = 0.151), but no significant effects for task (p = 0.358, η 2 = 0.020) or the interaction between group and task (p = 0.429, η 2 = 0.037) were found when adjusting for age and gender. As shown in Table 2 , both patients with TTC and HF showed an overall lower level of self-reported arousal compared with healthy controls, which remained significant when adjusting for age and gender. The other emotional subscales (distress and irritability) showed no significant between-subject or interaction effects (p >0.10, η 2 <0.060).
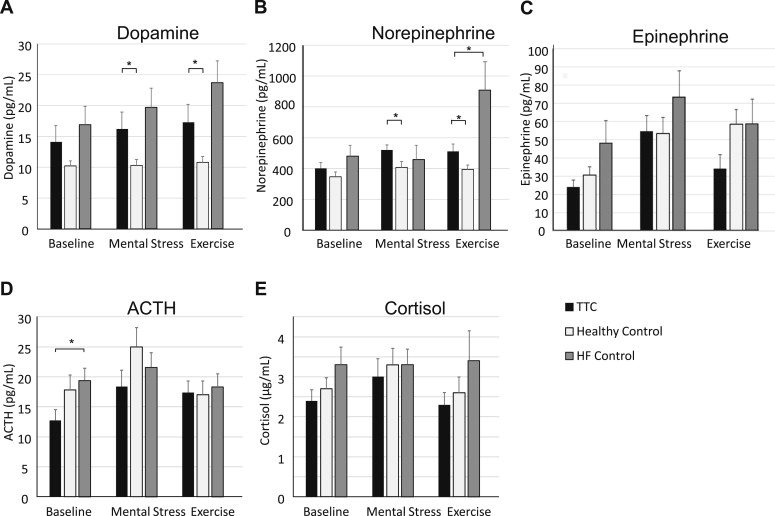
Neurohormonal responses are displayed in Figure 1 . Baseline levels were comparable between TTC and the 2 control groups for epinephrine, norepinephrine, dopamine, and cortisol. Baseline ACTH levels were lower in patients with TTC compared with HF (12.5 ± 6.6 vs 19.4 ± 8.6 pg/ml, p = 0.019). Mental stress produced significant increases in epinephrine, norepinephrine, and dopamine levels (p <0.02). During mental stress, norepinephrine (520.7 ± 125.5 vs 407.9 ± 155.3 pg/ml, p = 0.021) and dopamine (16.2 ± 10.3 vs 10.3 ± 3.9 pg/ml, p = 0.027) levels were significantly higher in patients with TTC compared with healthy controls. No differences were observed for epinephrine levels during mental stress (54.6 ± 32.4 vs 53.5 ± 35.8 pg/ml, p = 0.758). The magnitude of the dopamine response from baseline to mental stress was higher in patients with TTC than healthy controls (Δ = 2.2 ± 2.6 vs Δ = 0.03 ± 1.0 pg/ml, p = 0.004). The magnitude of norepinephrine and epinephrine increases from baseline was not significantly elevated in TTC versus controls. Repeated-measures analyses showed consistent results with significant increases with mental stress (within-subject effects p values <0.01, η 2 >0.200), a significant group × task interaction (F 2,35 = 5.12, p = 0.011, η 2 = 0.226) and group main effect (F 2,35 = 4.39, p = 0.020, η 2 = 0.200) for dopamine, and a group main effect for norepinephrine (F 2,35 = 4.54, p = 0.018, η 2 = 0.206). The group × task interaction for dopamine remained significant when adjusting for gender and age (p interaction = 0.035, η 2 = 0.184), but the patient group main effects for dopamine and norepinephrine were nonsignificant in these adjusted models (p >0.10, η 2 <0.090).