Electrophysiologic Studies
Jay D. Sengupta
I. INTRODUCTION.
Electrophysiologic studies (EPSs) are a specialized form of cardiac catheterization that help identify, characterize, and manage cardiac arrhythmias. Over the past 25 to 30 years, EPSs have advanced our knowledge of mechanisms of cardiac arrhythmias and revolutionized the way these arrhythmias are managed. The studies should be performed by trained clinicians with the help of skilled laboratory personnel in appropriately equipped laboratories. A joint task force of the American College of Cardiology and the American Heart Association in collaboration with Heart Rhythm Society has published guidelines outlining the accepted indications and the training required for personnel performing EPSs.
II. INDICATIONS.
The indications for EPSs can be divided into three broad categories: bradyarrhythmias, tachyarrhythmias, and syncope.
A. Bradyarrhythmias can be caused by sinus node dysfunction, atrioventricular (AV) nodal disease, or infranodal conduction system disease. EPSs for bradyarrhythmias are rarely necessary because the decision to implant a pacemaker depends primarily on correlation between symptoms and documented bradycardia or demonstration of severe bradycardia or prolonged pauses. EPSs should complement the clinical evaluation, conventional 12-lead electrocardiogram (ECG), and Holter or event monitor. EPSs can be of value in identifying disorders associated with adverse outcome, such as severe infranodal conduction system disease.
B. More often, EPSs may suggest bradycardia as the underlying disorder among patients with syncope of unknown cause. Thus, patients with syncope of uncertain cause may benefit from EPSs.
C. EPSs are of tremendous value in evaluating tachyarrhythmias. They are generally more successful in reproducing re-entrant cardiac rhythms than those caused by triggered activity or enhanced automaticity. Among patients with re-entrant tachyarrhythmias, EPSs are useful in documenting the presence of the anatomic or physiologic substrate responsible for the arrhythmia, defining electrical mechanism of the arrhythmia and its associated hemodynamic response, as well as guiding therapy. The response of the tachyarrhythmia during an EPS to various drugs or pacing maneuvers may also be helpful in further defining the underlying substrate and prognosis.
III. EQUIPMENT AND SETTING
A. The most important element in the performance of a safe and useful EPS is the presence of well-trained personnel. The presence of at least one trained physician and well-trained laboratory support personnel, including a nurse, as well as engineering assistance to maintain and repair the laboratory equipment is necessary. Personnel involved should be familiar with basic electrophysiologic and electropharmacologic principles, the indications for EPSs, and the various diagnostic and therapeutic modalities that can be used in the laboratory.
B. It is important that the laboratory be equipped with appropriate high-quality radiographic equipment.
C. Appropriate selection of tools is a very important aspect of the performance of a safe and cost-effective EPS. The minimum instrumentation required for a complete study is a stimulator, an amplifier, display monitors, reliable recording devices, and an external defibrillator.
1. The stimulator must be capable of burst pacing, delivery of at least three or four extra stimuli, synchronization to appropriate electric events during intrinsic or paced rhythms, and an adjustable current output. An appropriate unit should have a constant current source and minimal current leakage. It should also be relatively easy to manipulate.
2. The junction box connects the electrode catheters to the recording apparatus and the stimulator.
3. Recording is best achieved on solid media (e.g., CD or other optical media).
4. The presence of at least two functioning external defibrillators is extremely important, particularly during studies in which ventricular arrhythmias may be induced.
5. The presence of a cardiac surgical team in the same institution is not mandatory for routine EPS or simple radiofrequency (RF) ablation procedures. However, for more complex RF ablation procedures where full heparinization is used and where cardiac perforation is a potential complication, the presence of a cardiac surgical team allows for prompt, definitive therapy when surgical intervention is required.
D. Intracardiac signals are recorded using various electrode catheters.
1. The most common catheters used are quadripolar woven Dacron polyester or polyurethane. The distal poles of these catheters can be used for pacing.
2. For general purpose sensing and pacing in the atrium or ventricle, a nondeflectable catheter is usually sufficient. Deflectable catheters facilitate mapping and ablation by allowing more precise movement.
3. Interelectrode distance varies from 2 to 10 mm. Smaller interelectrode distance is useful for precise mapping and timing.
4. For most EPSs, bipolar recording is used. However, in some situations, especially during mapping of tachyarrhythmias, unipolar recording can be of value in localizing the earliest sites of activity.
IV. TECHNIQUES AND PROCEDURES
A. Preprocedure preparation
1. Before the patient is taken to the EPS laboratory, a discussion of the indications and proposed procedure is conducted with the patient, and informed consent is obtained.
2. For most indications, EPS is an elective procedure. The patient’s condition should be clinically stable at the time of the study. EPSs on patients who are unstable, including those with active, recent, or untreated coronary disease or those with clinical heart failure, carry much higher risk for complications.
3. Electrolytes, serum digoxin level, and bleeding measurements are checked and verified as being within the acceptable range.
4. Conscious sedation using a mild sedative (e.g., a benzodiazepine) and analgesic is administered.
5. The patient is attached to continuous ECG and blood pressure monitoring devices.
B. Access and catheter placement
1. The usual approach to inserting electrode catheters is through the femoral veins under local anesthesia, unless there is a clear contraindication to this approach, such as the presence of deep venous thrombosis or an inferior vena cava filter. In
the latter situations or when a coronary sinus (CS) catheter is difficult to insert, a superior vein approach may be used. We routinely utilize vascular ultrasound to directly visualize the femoral venipuncture, particularly when patients are on anticoagulation. This real-time visualization clarifies anatomic variants and enables us to avoid inadvertent arterial needlesticks and multiple passes that can lead to bleeding complications. Sheaths are then introduced into the vein over guidewires via the modified Seldinger technique.
the latter situations or when a coronary sinus (CS) catheter is difficult to insert, a superior vein approach may be used. We routinely utilize vascular ultrasound to directly visualize the femoral venipuncture, particularly when patients are on anticoagulation. This real-time visualization clarifies anatomic variants and enables us to avoid inadvertent arterial needlesticks and multiple passes that can lead to bleeding complications. Sheaths are then introduced into the vein over guidewires via the modified Seldinger technique.
2. Up to three introducers are placed in each femoral vein depending on the planned procedure. For patients with left-sided bypass tracks or left ventricular (LV) tachycardia, access to the left side of the heart is necessary. This can be achieved through the retrograde transaortic approach via an arterial access or by transseptal puncture via a femoral vein access. Systemic heparin is used for all left-sided procedures, and activated clotting time is monitored during the procedure to achieve adequate levels of anticoagulation.
3. For a complete EPS, three catheters are needed.
a. One catheter is placed in the high right atrium, preferably in the appendage or against the high lateral wall. Another is placed in the right ventricular (RV) apex, and the third is placed across the tricuspid valve to obtain a His electrogram.
b. To obtain a His electrogram, the electrode catheter is advanced into the right ventricle across the anterior septal portion of the tricuspid valve. Under gentle clockwise torque, the catheter is then slowly withdrawn to straddle the tricuspid valve. A high-frequency sharp deflection that precedes ventricular activation and follows septal atrial activation represents a His or proximal right bundle potential. If the catheter is drawn further, this sharp signal occurs slightly earlier. A satisfactory position of the His catheter is achieved when an atrial signal is recorded followed by the His potential and, finally, the ventricular potential is recorded via the same pair of electrodes.
4. In supraventricular tachycardia (SVT) studies, when a left-sided accessory pathway or left atrial origin is suspected, an octapolar or decapolar catheter may be placed in the CS rather than in the high right atrium. This more stable catheter position allows mapping of the left AV groove along the mitral annulus. Although the CS is easily entered from the superior venous approach, successful catheterization is expected in most attempts through the femoral approach as well. The catheter is placed in the CS with the proximal electrodes just inside the CS ostium.
C. Baseline assessment
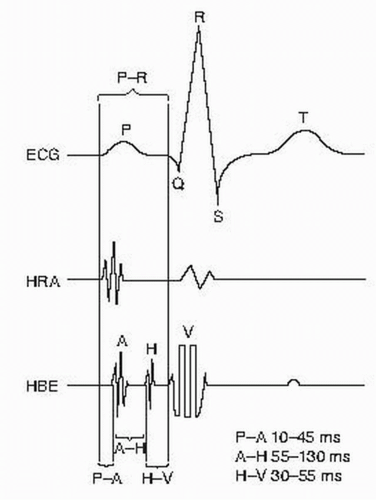
1. When all the catheters are in place, a baseline ECG (generally leads I, aVF, V1, and V6) and intracardiac electrograms are obtained (Fig. 53.1).
2. In general, cycle lengths rather than beats per minute are measured. The following measurements are made at baseline: sinus cycle length and PR, QRS, QT, AH, and HV intervals. To convert an arrhythmia’s rate from cycle length to beats per minute, divide 60,000 by the cycle length to obtain the arrhythmia rate in beats per minute.
a. The AH interval is measured from the onset of the local A deflection to the H deflection on the His electrogram.
b. The HV interval is measured from the H deflection on the His electrogram to the earliest ventricular activity in any lead (surface or intracardiac).
3. When measurements are made during pacing, it is important to measure from the resulting deflection rather than from the pacing artifact to avoid errors caused by latency (delay between the pacing artifact and activation at the recording site).
4. All measurements are recorded in milliseconds. Interpretation of baseline intervals is shown in Figure 53.2.
D. Programmed stimulation
1. After baseline measurements are made, programmed stimulation is performed. The protocol used depends on the indication for the study and varies among institutions. During programmed stimulation, the hemodynamic response of the patient to pacing and induced tachycardia is closely monitored. For example, rapid ventricular pacing in patients with structural heart disease may result in transient hypotension. If this occurs, pacing should be limited in duration and adequate time between pacing drive trains should be allowed for hemodynamic recovery.
2. Pacing stimuli are usually delivered at 1 or 2 millisecond pulse width and at twice diastolic pacing thresholds. This is important during ventricular stimulation because pacing at higher outputs increases the risk of inducing nonclinical rhythms. There are two main types of programmed stimulation: burst pacing and the extra stimulus technique.
a. Burst pacing involves continuous pacing at rates faster than the patient’s intrinsic rate.
b. In the extra stimulus technique, premature beats are introduced either during intrinsic rhythm (sensed extra stimuli) or after a paced drive train (paced extra stimuli). Extra stimulus techniques are useful in evaluating refractory periods of the AV node, atrial tissue, ventricular tissue, and accessory pathways. It is possible to evaluate infranodal conduction system refractory periods with atrial or ventricular stimulation. Extra stimulus techniques are also useful in inducing, terminating, and identifying re-entrant arrhythmias.
(1) In the sensed extra stimulus technique, a single extra stimulus (S2) is introduced initially with a coupling just below the intrinsic rate. The coupling interval is reduced progressively by 10 to 20 milliseconds until the premature stimulus no longer captures. A pause of 2 to 5 seconds is allowed between stimulation sequences. Multiple extra stimuli (S3, S4) can be added if necessary and the sequence repeated.
(2) In the paced extra stimulus technique, a drive train of 6 to 10 beats at a fixed cycle length is followed by the premature beat. The drive train cycle length (S1S1) usually ranges from 350 to 800 milliseconds (most frequently 400 to 600 milliseconds) but depends on the resting heart rate. When this technique is used, testing at two drive train cycle lengths is recommended. The premature stimulus (S2) is introduced with a coupling interval just below the S1S1. The coupling interval of the premature stimulus is decreased progressively by 10 to 20 milliseconds until it no longer captures. The longest coupling interval (S1S2) that does not capture the myocardium is the absolute refractory period. S3 and S4 are added if necessary. This protocol can be varied depending on the indication and operator preference.
3. Continuous monitoring and recording of external and intracardiac electrograms is maintained throughout programmed stimulation. When a particular event such as a tachycardia occurs, stimulation is stopped and the event evaluated. The operator should be ready to respond to the event appropriately, depending on the effect that the event has on the patient. For example, induction of a sustained tachycardia may result in severe hypotension, angina, or loss of consciousness. In such circumstances, expeditious termination of the tachycardia is indicated through overdrive pacing or cardioversion. The operator should also be ready to perform pacing or other maneuvers to further assess the mechanisms and reentrant circuit of the induced tachycardia.
V. ATRIAL STIMULATION
A. An atrial study is an integral part of EPSs. The only time an atrial study is not performed is in the presence of persistent atrial fibrillation.
1. Burst atrial pacing at incremental rates causes slowing of AV nodal conduction (a process known as decremental conduction) and can induce tachycardia, including AV node re-entry tachycardia and AV re-entrant tachycardia. Other forms of tachycardia unrelated to AV nodal conduction can also be induced, such as atrial flutter, atrial fibrillation, atrial tachycardia, and certain forms of idiopathic ventricular tachycardia.
2. Burst pacing is performed by means of continuous pacing (e.g., 10 to 20 stimuli) at a fixed cycle length starting at 100 milliseconds below the baseline cycle length. Repeat burst pacing is performed at progressively shorter cycle lengths until 1:1 conduction through the AV node is no longer maintained. The shortest cycle length showing consistent 1:1 conduction through the AV node is recorded. This is related to the effective refractory period of the AV node, which is the longest A1-A2 interval that fails to be conducted to the His bundle. Another interval that can be measured is the functional refractory period of the AV node: it is defined as the shortest output interval from the AV node to the His bundle, given any input signal.
3. If a patient is believed to have atrial flutter or atrial tachycardia, repeat burst pacing at even shorter cycle lengths is performed until 1:1 atrial capture is no longer maintained.
B. Another form of atrial stimulation that is performed is paced extra stimulus.
1. The effect of atrial premature beats on the AH interval is assessed. The normal response of the AH interval is to progressively prolong with shorter A1A2 coupling. This is a direct demonstration of the normal decremental conduction properties of the AV node.
2. At a critical A1A2, the AV node fails to conduct, and on the His electrogram an atrial signal is seen without a His or ventricular deflection. This indicates that a block has occurred in the AV node. It is important to continue stimulation until the atrial refractory period is reached because a gap phenomenon may occasionally exist as a result of dual AV nodal pathways.
3. The gap phenomenon is demonstrated by apparent achievement of the AV nodal refractory period followed by resumption of conduction at shorter A1A2 coupling intervals. It reflects functional differences in conduction velocity or refractoriness in several regions of the AV junction.
4. If narrow complex tachycardia is induced, it is evaluated with regard to type, mechanism, response to maneuvers, and method of termination (see Section IX.B.3).
C. Sinus node evaluation. For patients who may have underlying sinus node dysfunction, sinus node tests are sometimes performed.
1. Sinus node recovery time (SNRT) is evaluated through burst pacing at various cycle lengths in the atrium for 30 to 60 seconds, followed by abrupt termination of pacing. SNRT is the escape interval between the last paced atrial beat and the first atrial recovery beat. A corrected sinus node recovery time (CSNRT) is calculated by means of subtracting the baseline sinus cycle length from SNRT. A normal value for CSNRT is < 550 milliseconds. SNRT is used to evaluate the automaticity mechanism of the sinus node.
2. Sinoatrial conduction time (SACT) is a combined measure of conduction in the atrial tissue that includes the area of the sinus node and sinus node automaticity. The assumptions are first that the conduction times into and out of the sinus node are equal, second that the pacing train does not alter the automaticity of the sinus node, and third that the pacemaking site does not change after premature stimulation. The SACT is measured with one of two methods.
a. In the Strauss method, a sensed premature atrial beat is used to reset the sinus node, and the return cycle length after the premature beat is measured. The basic cycle length is subtracted from the return cycle length, leaving out
the time necessary to penetrate and leave the sinus nodal tissue. SACT is one-half this interval.
the time necessary to penetrate and leave the sinus nodal tissue. SACT is one-half this interval.
b. In the method proposed by Narula, the same measurements are obtained after pacing for eight beats at a rate slightly faster than the sinus rate. The upper range of SACT is 100 to 120 milliseconds.
The sensitivity of each individual (SACT and SNRT) test in diagnosing sinus node dysfunction is approximately 50% when used alone and 65% when combined. The specificity of the two combined tests is 88%, which gives the test a high positive predictive value. However, because of its low sensitivity, a normal test does not exclude sinus node disease.
VI. VENTRICULAR STIMULATION
A. Ventricular stimulation is performed in evaluations of suspected SVTs or ventricular tachyarrhythmias. Some SVTs, such as unusual forms of AV nodal re-entry, may be more easily induced with ventricular stimulation. To further characterize the tachycardia, the response of the tachycardia to premature ventricular beats can be assessed.
1. When ventricular stimulation is performed for the evaluation of ventricular or wide complex tachyarrhythmias, pacing at two or more sites maybe necessary. These sites are typically the RV apex and the RV outflow tract.
2. Before programmed stimulation is begun, pacing thresholds are determined, and the output of the pacing stimulus is set to twice the diastolic capture threshold. Higher outputs or coupling intervals shorter than 200 milliseconds may cause induction of nonclinical arrhythmias.
B. Burst pacing in the right ventricle is one of two techniques used when assessing retrograde ventriculoatrial (VA) conduction in the evaluation of SVT.
1. The presence of retrograde atrial activation is documented, and a sequence or pattern of atrial activation is evaluated.
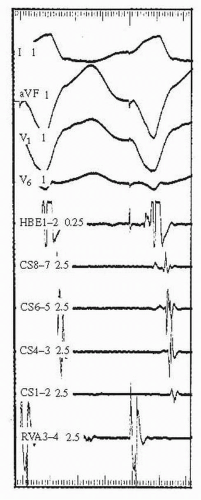
2. The earliest atrial activity during retrograde conduction via the AV node is typically recorded on the His electrogram (see Fig. 53.2). This indicates that retrograde conduction has proceeded through the AV node fast pathway. Absence of VA conduction, with rare exceptions (e.g., the Mahaim type of accessory pathway which only conducts in the antegrade direction), excludes the presence of a bypass track. The presence of eccentric atrial activation (late atrial activation on the His electrogram; see Fig. 53.3) suggests the presence of a retrogradely conducting bypass track.
3. For some patients with no evidence of retrograde VA conduction, infusion of low doses of isoproterenol or a small dose of atropine (0.5 mg) restores this property to the AV node.
4. The shortest paced cycle length capable of 1:1 conduction to the atrium is documented.
C. Premature ventricular stimulation is another technique used to evaluate retrograde conduction properties of the heart.
1. If retrograde conduction is present, the refractory periods of the conducting pathways are determined with the extra stimulus technique.
2. In patients with retrograde VA conduction through the AV node, conduction block of a ventricular premature beat frequently occurs in the His-Purkinje system rather than in the AV node. His-Purkinje conduction block is more likely to occur at long drive trains. Such drive trains therefore are more likely to induce AV re-entry tachycardia (using a bypass track) by facilitating retrograde His-Purkinje block and allowing a retrograde conducted beat through the pathway to propagate antegrade through the AV node.
D. In patients being evaluated for ventricular arrhythmias, programmed stimulation with extra stimuli is the initial technique used. Pacing at two drive train cycle lengths (e.g., 600 and 400 milliseconds) is performed with single, double, and triple extra stimuli. Simultaneous atrial pacing at the same drive train cycle length is sometimes necessary to avoid competition from the intrinsic atrial pacemaker.
E. Like the A1A2 technique described earlier, V2 is introduced at progressively shorter coupling intervals (V1V2) until V2 no longer captures (ventricular refractory period). Then V2 is set at a coupling interval longer than the refractory period and V3 is introduced at progressively shorter coupling intervals until it no longer captures. The use of triple extra stimuli (V3V4) is usually reserved for patients being evaluated for ventricular arrhythmias.
A pause of 4 to 5 seconds is allowed after each cycle to assess response and for the patient to recover after ventricular pacing. An increase in the number of extra stimuli increases the sensitivity of the study in reproducing clinical arrhythmias, but at the cost of a lower specificity due to initiation of polymorphic ventricular tachycardia or ventricular fibrillation.
If programmed stimulation with ventricular extra stimuli does not induce ventricular tachycardia in a patient at very high risk, other techniques may be used. One is burst pacing in the ventricle. A series of 10 paced ventricular beats are introduced at a constant cycle length. The paced cycle length is then decreased by 50 to 100 milliseconds in successive bursts until reaching within 50 milliseconds of the predicted refractory period of the right ventricle, when the decrements proceed at 10-millisecond intervals until 1:1 capture is no longer maintained. Burst pacing in the atrium can at times induce idiopathic LV tachycardia in susceptible persons.
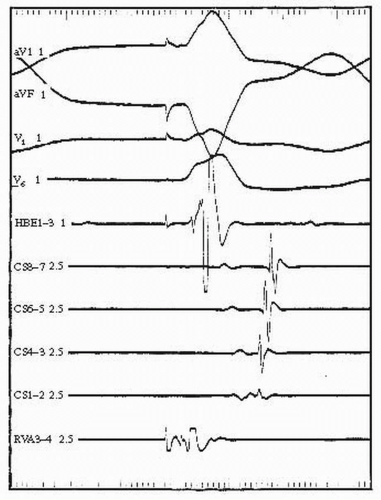
F. In some patients, particularly those with underlying dilated cardiomyopathy, bundle branch re-entry (BBR) tachycardia (see Fig. 53.4) may be induced.
1. This type of tachycardia usually involves the right bundle branch as the antegrade limb and the left bundle branch as the retrograde limb of the re-entrant circuit. It is usually a rapid and hemodynamically unstable tachycardia.
2. Because His bundle refractoriness increases after a pause, a short-long-short stimulation sequence can be used to cause retrograde block in the right bundle so that the paced stimulus can conduct retrograde up the left bundle branch and possibly initiate tachycardia if the right bundle branch is no longer refractory for antegrade conduction.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree