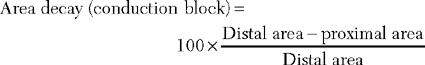Chapter 4 Electrodiagnostic Findings in Neuromuscular Disorders
Details of the electrodiagnostic findings in various neuromuscular disorders are outlined within the case studies in this book. The following is a brief summary of these findings in the most common neuromuscular disorders.
FOCAL MONONEUROPATHIES
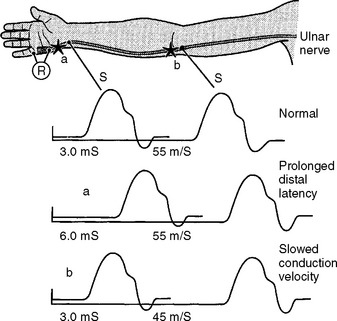
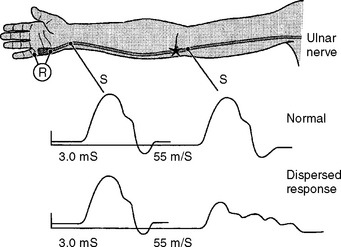
Demyelinative Mononeuropathy
There are several limitations to the definitive diagnosis of demyelinative conduction block:
Table 4-1 Electrodiagnosis of Conduction Block
| Definite in Any Nerve* 1. ≥50% drop in CMAP amplitude with a 15% prolongation of CMAP duration and with ≥50% drop in CMAP area |
| Possible in Median, Ulnar, and Peroneal Nerves Only |
* Caution should be taken in evaluating the tibial nerve, where stimulation at the knee can be submaximal, resulting in 50% or at times greater than 50% drop in amplitude and area, especially in overweight and very tall patients.
Axon-Loss Mononeuropathy
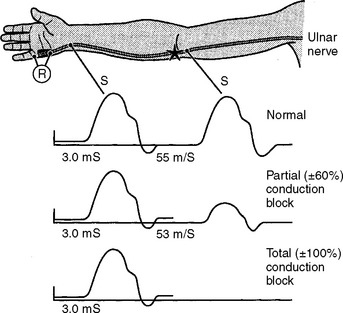
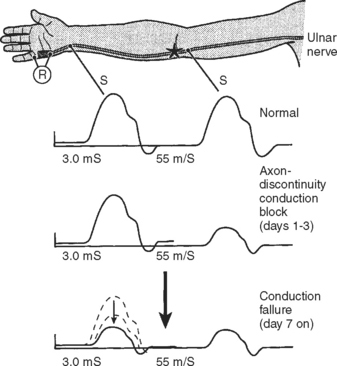
Following acute focal axonal damage, the distal nerve segment undergoes wallerian degeneration. However, early after axonal transaction, the distal axon remains excitable. Hence, stimulation distal to the lesion elicits a normal CMAP, whereas proximal stimulation elicits an absent response (complete conduction block) when the lesion is total and reduced CMAP amplitude (partial conduction block) when the lesion is incomplete (Figure 4-4). In an attempt to distinguish this pattern from a demyelinative conduction block, some refer to this pattern as an axonal noncontinuity, early axon loss, or axon discontinuity conduction block.
Wallerian degeneration of the axons distal to the nerve lesions is completed in 7–11 days. In the first 1–2 days, the distal CMAP and SNAP are normal. The distal CMAP amplitude then decreases and reaches its nadir in 5–6 days, while the distal SNAP amplitude lags slightly behind. It starts declining in amplitude after 4–5 days and reaches its nadir in 10–11 days (Figure 4-5). The earlier decline of the CMAP amplitude comparing to the SNAP amplitude following axon-loss nerve lesion is likely related to the early neuromuscular transmission failure that affects the recording of the CMAP amplitudes only. This is supported by the fact that MNAPs, recorded directly from nerve trunks, follow the time course of SNAPs.
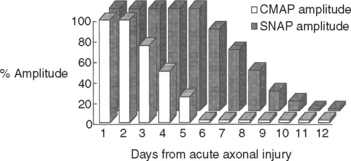
Figure 4-5 Effect of wallerian degeneration on distal CMAP and SNAP amplitudes following acute nerve lesion.
On motor NCS, a conduction block is present soon after axonal injury. However, as the distal axons undergo wallerian degeneration, this is replaced by unelicitable or low CMAP amplitudes with both distal and proximal stimulations corresponding to complete or partial motor axonal loss lesions respectively (see Figure 4-4). At this time, the distal CMAP amplitude is a reliable semiquantitative estimate of the amount of axonal loss in peripheral nerve lesions. In the chronic phases of partial axonal nerve lesions with reinnervation via collateral sprouting, the CMAP may improve to reach normal or near normal values giving a false indication of a milder degree of original axonal loss.
In contrast to demyelinating or mixed mononeuropathies, pure axon-loss peripheral nerve lesions cannot be localized by NCSs when studied after the completion of wallerian degeneration, since they are not associated with focal conduction slowing or block. The identification of conduction block in the early days of axonal loss is extremely helpful in localizing a peripheral nerve injury. Waiting for the completion of wallerian degeneration results in diffusely low or unevoked CMAPs (regardless of stimulation site), which does not allow for accurate localization of the injury site. Localizing a purely axon-loss mononeuropathy after the completion of wallerian degeneration depends on needle EMG, with principles that are similar to manual muscle strength testing used during the neurological examination. Typically, the needle EMG reveals neurogenic changes (fibrillation potentials, reduced MUAP recruitment, chronic neurogenic MUAP morphology changes) that are limited to muscles innervated by the injured nerve distal to the site of the lesion (Figure 4-6). In contrast, muscles innervated proximal to the lesion remain normal. Unfortunately, attempting to localize axon loss lesions solely by needle EMG has several shortcomings that may result in poor localization or, sometimes, mislocalization of the site of the nerve lesion. These include the following scenarios:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree