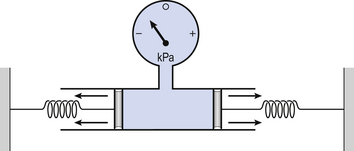
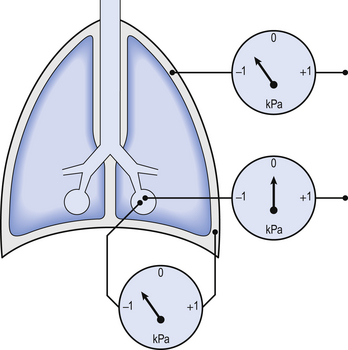
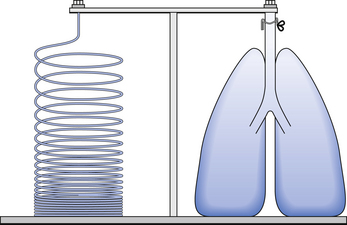
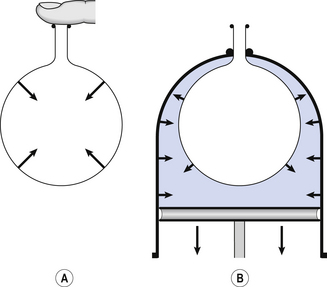
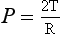3 The most commonly used model of lung inflation is a toy balloon. Many of the principles that follow can be demonstrated by this model. For example, if you inflate a balloon and prevent the air escaping by blocking the neck with your finger (Fig. 3.1A), the elastic recoil of the balloon will be proportional to its elastance (1/compliance, see below) and will produce a recoil pressure. The pressure inside the balloon will be the same throughout if no flow is taking place into or out of the balloon. These observations demonstrate important principles of lung function. Fig. 3.1 (A) Balloon demonstrating elastic recoil. (B) Physiological model of the respiratory system. An even more physiological model of the respiratory system can be made by suspending a balloon in a jar with a piston at its base, like a large syringe (Fig. 3.1B). In this case the balloon represents the lungs, the jar represents the chest wall and the piston represents the diaphragm. Lowering the piston reduces the pressure round the balloon (intrapleural pressure) and causes it to inhale. For an object to be stretched or in some other way distorted it must be subjected to a force. In the case of a three-dimensional object this force may be pressure. In our simple model of breathing (Fig. 3.1A), inspiration would be inflation of the balloon and expiration deflation. The pressure that brings about inflation in Figure 3.1A would be applied to the inside. There is a pressure gradient from inside the balloon to outside. The other, more complicated, way for us to inflate the balloon would be to reduce the pressure outside it using the jar and plunger (Fig. 3.1B): again there is a pressure gradient from inside to outside the balloon, and this is the way we inflate our lungs. Another way of visualizing what is happening in the space between the lungs and chest wall is to imagine a syringe with two plungers being pulled in opposite directions (Fig. 3.2). You can see from such a model that intrapleural pressure is negative with respect to atmospheric pressure. What is not immediately obvious is that intrapleural pressure is also negative with respect to air pressure within the alveoli, because the alveoli are connected to the atmosphere by a system of open tubes, the bronchial tree (Fig. 3.3). This means a hole made between either the atmosphere or the alveoli and the intrapleural space will allow the pressure surrounding the lung to rise and the lung to collapse: this dangerous condition is called a pneumothorax. Because the lungs are to some extent suspended from the trachea and rest on the diaphragm, they behave like a child’s ‘slinky’ (a very soft spring), held at one end and supported from underneath. Gravity causes the spring or lungs to slump under their own weight (Fig. 3.4).
ELASTIC PROPERTIES OF THE RESPIRATORY SYSTEM
Introduction
Intrapleural pressure (Ppl)
ELASTIC PROPERTIES OF THE RESPIRATORY SYSTEM