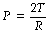Chapter 3 Elastic forces and lung volumes
 Inward elastic recoil of the lung opposes outward elastic recoil of the chest wall, and the balance of these forces determines static lung volumes.
Inward elastic recoil of the lung opposes outward elastic recoil of the chest wall, and the balance of these forces determines static lung volumes. Surface tension within the alveoli contributes significantly to lung recoil, and is reduced by the presence of surfactant, though the mechanism by which this occurs is poorly understood.
Surface tension within the alveoli contributes significantly to lung recoil, and is reduced by the presence of surfactant, though the mechanism by which this occurs is poorly understood. Compliance is defined as the change in lung volume per unit change in pressure gradient, and may be measured for lung, thoracic cage or both.
Compliance is defined as the change in lung volume per unit change in pressure gradient, and may be measured for lung, thoracic cage or both.The last three may be grouped together as non-elastic resistance or respiratory system resistance; they are discussed in Chapter 4. They are measured while gas is flowing within the airways, and work performed in overcoming this ‘frictional’ resistance is dissipated as heat and lost.
This chapter is concerned with the elastic resistance afforded by lungs (including the alveoli) and chest wall, which will be considered separately and then together. When the respiratory muscles are totally relaxed, these factors govern the resting end-expiratory lung volume or FRC, and therefore lung volumes will also be considered in this chapter.
Elastic Recoil of the Lungs
Compliance may be described as static or dynamic depending on the method of measurement (page 38). Static compliance is measured after a lung volume has been held at a fixed volume for as long as is practicable, while dynamic compliance is usually measured in the course of normal rhythmic breathing. Elastance is the reciprocal of compliance and is expressed in kilopascals per litre. Stiff lungs have a high elastance.
The Nature of the Forces Causing Recoil of the Lung
For many years it was thought that the recoil of the lung was due entirely to stretching of the yellow elastin fibres present in the lung parenchyma. In 1929, von Neergaard (page 243) showed that a lung completely filled with and immersed in water had an elastance that was much less than the normal value obtained when the lung was filled with air. He correctly concluded that much of the ‘elastic recoil’ was due to surface tension acting throughout the vast air/water interface lining the alveoli.
where P is the pressure within the bubble (dyn.cm−2), T is the surface tension of the liquid (dyn.cm−1) and R is the radius of the bubble (cm). In coherent SI units (see Appendix A), the appropriate units would be pressure in pascals (Pa), surface tension in newtons/metre (N.m−1) and radius in metres (m).
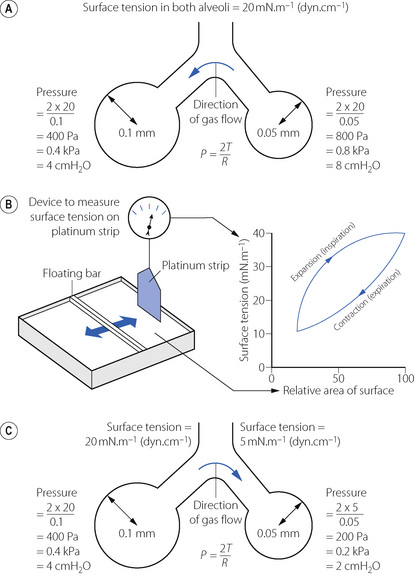
On the left of Figure 3.1A is shown a typical alveolus of radius 0.1 mm. Assuming that the alveolar lining fluid has a normal surface tension of 20 mN.m−1 (= 20 dyn.cm−1), the pressure within the alveolus will be 0.4 kPa (4 cmH2O), which is rather less than the normal transmural pressure at FRC. If the alveolar lining fluid had the same surface tension as water (72 mN.m−1), the lungs would be very stiff.
The alveolus on the right of Figure 3.1A has a radius of only 0.05 mm and the Laplace equation indicates that, if the surface tension of the alveolus is the same, its pressure should be double the pressure in the left-hand alveolus. Thus gas would tend to flow from smaller alveoli into larger alveoli and the lung would be unstable which, of course, is not the case. Similarly, the retractive forces of the alveolar lining fluid would increase at low lung volumes and decrease at high lung volumes, which is exactly the reverse of what is observed. These paradoxes were clear to von Neergaard and he concluded that the surface tension of the alveolar lining fluid must be considerably less than would be expected from the properties of simple liquids and, furthermore, that its value must be variable. Observations 30 years later confirmed this when alveolar extracts were shown to have a surface tension much lower than water and which varied in proportion to the area of the interface.1 Figure 3.1B shows an experiment in which a floating bar is moved in a trough containing an alveolar extract. As the bar is moved to the right, the surface film is concentrated and the surface tension changes as shown in the graph on the right of the figure. During expansion, the surface tension increases to 40 mN.m−1, a value which is close to that of plasma but, during contraction, the surface tension falls to 19 mN.m−1, a lower value than any other body fluid. The course of the relationship between pressure and area is different during expansion and contraction, and a loop is described.
The consequences of these changes are very important. In contrast to a bubble of soap solution, the pressure within an alveolus tends to decrease as the radius of curvature is decreased. This is illustrated in Figure 3.1C where the right-hand alveolus has a smaller diameter and a much lower surface tension than the left-hand alveolus. Gas tends to flow from the larger to the smaller alveolus and stability is maintained.
The Alveolar Surfactant
The low surface tension of the alveolar lining fluid and its dependence on alveolar radius are due to the presence of a surface-active material known as the surfactant.2 Some 90% of surfactant consists of lipids, the remainder being proteins and small amounts of carbohydrate. Most of the lipid is phospholipid, of which 70–80% is dipalmitoyl phosphatidyl choline (DPPC), the main constituent responsible for the effect on surface tension. The fatty acids are hydrophobic and generally straight, lying parallel to each other and projecting into the gas phase. The other end of the molecule is hydrophilic and lies within the alveolar lining fluid. The molecule is thus confined to the surface where, being detergents, they lower surface tension in proportion to the concentration at the interface.
Around 10% of surfactant obtained from bronchio-alveolar lavage is protein, most of which are contaminating serum proteins such as albumin and globulin. Approximately 2% of surfactant by weight consists of surfactant proteins (SP), of which there are four types labelled A–D.3,4 SP-B and SP-C are small proteins that are vital to the stabilisation of the surfactant mono-layer (see below); a congenital lack of SP-B results in severe and progressive respiratory failure.4,5 SP-A, and to a lesser extent SP-D, are involved in the control of surfactant release and possibly in preventing pulmonary infection (see below).6
Synthesis of surfactant. Surfactant is both formed in and liberated from the alveolar epithelial type II cell (page 23). The lamellar bodies (see Figure 2.10) contain stored surfactant that is released into the alveolus by exocytosis in response to high volume lung inflation, increased ventilation rate or endocrine stimulation. After release surfactant initially forms areas of a lattice structure termed tubular myelin, which is then reorganised into mono- or multi-layered surface films. This conversion into the functionally active form of surfactant is believed to be critically dependent on surfactant proteins B and C (see below).4,6 The alveolar half life of surfactant is 15–30 hours with most of its components being recycled by type II alveolar cells. Surfactant protein-A is intimately involved in controlling the surfactant present in the alveolus with type II alveolar cells having SP-A surface receptors, stimulation of which exerts a negative feedback on surfactant secretion and increases re-uptake of surfactant components into the cell.
Action of surfactant. To maintain the stability of alveoli as shown in Figure 3.1, surfactant must alter the surface tension in the alveoli as their size varies with inspiration and expiration. A simple explanation of how this occurs is that during expiration, as the surface area of the alveolus diminishes, the surfactant molecules are packed more densely and so exert a greater effect on the surface tension, which then decreases as shown in Figure 3.1b. In reality, the situation is considerably more complex, and at present poorly elucidated.4 The classical explanation, referred to as the ‘squeeze out’ hypothesis, is that as a surfactant monolayer is compressed, the less stable phospholipids are squeezed out of the layer, increasing the amount of stable DPPC molecules which have the greatest effect in reducing surface tension.7 Surfactant phospholipid is also known to exist in vivo in both monolayer and multilayer forms,3 and it is possible that in some areas of the alveoli the surfactant layer alternates between these two forms as alveolar size changes during the respiratory cycle. This aspect of surfactant function is entirely dependent on the presence of SP-B, a small hydrophobic protein, which can be incorporated into a phospho-lipid monolayer, and SP-C, a larger protein with a hydrophobic central portion allowing it to span a lipid bilayer.4 When alveolar size reduces and the surface film is compressed, SP-B molecules may be squeezed out of the lipid layer so changing its surface properties, while SP-C may serve to stabilise bilayers of lipid to act as a reservoir from which the surface film re-forms when alveolar size increases.
Other effects of surfactant. Pulmonary transudation is also affected by surface forces. Surface tension causes the pressure within the alveolar lining fluid to be less than the alveolar pressure. Since the pulmonary capillary pressure in most of the lung is greater than the alveolar pressure (page 420), both factors encourage transudation, a tendency that is checked by the oncotic pressure of the plasma proteins. Thus the surfactant, by reducing surface tension, diminishes one component of the pressure gradient and helps to prevent transudation.
Surfactant also plays an important part in the immunology of the lung.2,8,9 The lipid component of surfactant has anti-oxidant activity, so may attenuate lung damage from a variety of causes, and also suppresses some groups of lymphocytes so theoretically protecting the lungs from auto-immune damage. In-vitro studies have shown that SP-A or SP-D can bind to a wide range of pulmonary pathogens including viruses, bacteria, fungi, Pneumocystis carinii, and Mycobacterium tuberculosis. Acting via specific surface receptors, both SP-A and SP-D activate alveolar neutrophils and macrophages, and enhance the phagocytic actions of the latter during lung inflammation.9
Alternative Models to Explain Lung Recoil
Treating surfactant-lined alveoli as bubbles that obey Laplace’s law has aided the understanding of lung recoil in health and disease for many decades (page 243). This ‘bubble model’ of alveolar stability is not universally accepted,10,11 and evidence is mounting that the real situation is more complex. Arguments against the bubble model include:
 in theory, differing surface tensions in adjacent alveoli cannot occur if the liquid lining the alveoli is connected by a continuous liquid layer
in theory, differing surface tensions in adjacent alveoli cannot occur if the liquid lining the alveoli is connected by a continuous liquid layer when surfactant layers are compressed at 37°C multilayered ‘rafts’ of dry surfactant form, though inclusion of surfactant proteins reduces this physico-chemical change
when surfactant layers are compressed at 37°C multilayered ‘rafts’ of dry surfactant form, though inclusion of surfactant proteins reduces this physico-chemical change alveoli are not shaped like perfect spheres with a single entrance point – they are variable polyhedrons with convex bulges in their walls where pulmonary capillaries bulge into them (Figure 2.7).
alveoli are not shaped like perfect spheres with a single entrance point – they are variable polyhedrons with convex bulges in their walls where pulmonary capillaries bulge into them (Figure 2.7).Two quite different alternative models have been proposed:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree