Ivabradine is a specific heart rate-lowering antianginal agent that was evaluated in a clinical development program involving approximately 3,000 patients with stable coronary artery disease, most with angina pectoris. We analyzed the pharmacokinetics, efficacy (evaluated by exercise tolerance testing), safety, and effects on glucose metabolism of ivabradine in patients with diabetes mellitus (DM) in this program. Most analyses included data from 535 patients with DM, approximately 18% of the overall patient sample. Patients with DM were older, more likely to be women, and more likely to have more severe angina pectoris than patients without DM. The pharmacokinetics of ivabradine did not differ in patients with DM versus those without DM. A reduction in the heart rate at rest with ivabradine was similar in those with (15.2%) and without (15.7%) DM. At baseline, the exercise capacity tended to be lower in the patients with DM, but the improvements in most exercise tolerance measures with ivabradine treatment were similar in patients with and without DM. No special safety concerns were associated with ivabradine in those with DM. The rates of sinus bradycardia and visual disturbances, known to be related to the action of ivabradine, showed no relative increase in the patients with DM. Ivabradine treatment was not associated with adverse effects on glucose metabolism. In conclusion, ivabradine was effective in preventing angina in patients with DM and was not associated with particular safety concerns or adverse effects on glucose metabolism. Ivabradine represents an attractive alternative to β blockers in patients with stable angina pectoris and DM.
Ivabradine is a specific heart rate-lowering antianginal agent that inhibits the I f current, the primary modulator of spontaneous diastolic depolarization (the pacemaker current) in the sinoatrial node. At therapeutic concentrations, ivabradine has no appreciable action on other cardiac or vascular ion channels and has no direct effects on myocardial contractility, intracardiac conduction, or ventricular repolarization in humans. The antianginal and antimyocardial-ischemic efficacy of ivabradine has been demonstrated in randomized, controlled clinical trials and is not inferior to the β blocker atenolol or the calcium channel blocker amlodipine. Ivabradine is now recommended in European guidelines for the prevention of stable angina pectoris. The efficacy and safety of ivabradine were evaluated in a clinical development program that involved approximately 3,000 ivabradine-treated patients with stable coronary artery disease (CAD), more than 90% with angina. However, the drug’s effects specifically in patients with diabetes mellitus (DM) have not been reported. The present analysis assessed the pharmacokinetics, efficacy, and effects on glucose metabolism of ivabradine in patients with DM, drawn from the data in the clinical development program.
Methods
Data were drawn from the 8 multicenter, randomized, double-blind, controlled trials designed to assess the efficacy and safety of ivabradine during its clinical development before regulatory approval in Europe ( Table 1 ). In the large efficacy and safety studies (studies 1 to 6), male or female outpatients with chronic stable angina pectoris and documented CAD were treated for ≥3 months. The exclusion criteria included important heart disease other than CAD, Prinzmetal’s or microvascular angina, known high-grade left main CAD, New York Heart Association class III or IV heart failure, atrial fibrillation or flutter, pacemaker or implanted defibrillator, second- or third-degree atrioventricular block, heart rate at rest <50 beats/min or sick sinus syndrome, and symptomatic hypotension or uncontrolled hypertension (systolic blood pressure >180 mm Hg or diastolic blood pressure >100 mm Hg). For the efficacy studies (studies 1 to 4), the patients were required to have 2 symptom-limited exercise tolerance tests (ETTs) with positive findings showing relative stability for the time to the onset of myocardial ischemia (1-mm ST-segment depression). In study 7, patients were required to have documented CAD and to have been treated for congestive heart failure, New York Heart Association class II, for ≥6 months, with a left ventricular ejection fraction of 30% to 45%. In study 8, patients were required to have documented CAD, but angina was not required for inclusion. The DM subgroup in each analysis included all patients with a medical history of DM of any type and severity, as reported by the investigator.
| Study | Description, Reference | Type | Patients (n) | Treatment Group | ETT Type | Treatment Duration |
|---|---|---|---|---|---|---|
| 1 | Double-blind dose-ranging study | Stable angina | 360 |
| Bicycle | 2 weeks |
| 2 | Double-blind efficacy study, dose increase at 1 months | Stable angina | 939 |
| Treadmill | 4 months |
| 3 | Double-blind efficacy study, on top of amlodipine 10 mg once daily | Stable angina | 728 |
| Treadmill | 3 months |
| 4 | Double-blind efficacy study | Stable angina | 1,195 |
| Bicycle | 3 months |
| 5 | Double-blind safety study | Stable angina | 318 |
| None | 1 year |
| 6 | Double-blind safety study | Stable angina | 386 |
| None | 1 year |
| 7 | Double-blind safety and efficacy study | CAD + CHF | 65 |
| 6-minute walk test | 3 months |
| 8 | Double-blind safety study, escalating doses | CAD | 78 |
| None | 1 wk/dose |
In all studies, the use of short-acting nitrates for angina relief was permitted as required; however, the ETT could not be performed within 2 hours of the patient taking nitrates. In the efficacy studies, drugs that could prevent angina were not permitted, including β blockers, calcium channel blockers, long-acting nitrates, potassium channel openers, molsidomine, and trimetazidine (except if mandated by the study protocol as active comparators or as background therapy). Drugs that could affect the interpretation of the electrocardiographic changes were also precluded, including antiarrhythmic agents, digitalis, amiodarone, and monoamine oxidase inhibitors. A wider range of drugs was permitted in the safety studies. Most drugs were allowed, with the exception of agents that alter the heart rate, including β blockers and nondihydropyridine calcium channel blockers, and drugs with known or suspected interactions with ivabradine, including antifungal azole derivatives and macrolide antibiotics or protocol-mandated comparator drugs.
All patients gave written informed consent, and the study protocols were reviewed by the relevant independent ethics committees. The studies were conducted in accordance with the principles of the Declaration of Helsinki, 1964, and later amendments.
In the efficacy studies, exercise capacity was evaluated by either treadmill or bicycle ETTs ( Table 1 ) with 12-lead electrocardiographic recording (for details, see Borer et al , Tardif et al, and Ruzyllo et al ). In the treadmill ETT (studies 2 and 3), a modified Bruce protocol was used. When bicycle ergometry was used, the initial workload was 30 W, increased by 30 W every 2 minutes (study 1), or 40 W increased by 10 W every minute (study 4). The ETT parameters of total exercise duration, interval to 1-mm ST-segment depression, and interval to angina onset were recorded. The electrocardiographic printouts were read at a central core facility by physicians unaware of the treatment allocation and patient identification. The heart rate at rest was measured by electrocardiography at the study visits. All patients kept a weekly angina diary from which the frequency of angina attacks was calculated. In all studies, timed blood samples were taken from a subset of ivabradine-treated patients for pharmacokinetic analysis of ivabradine and its active metabolite, S 18982. Blood concentrations of hemoglobin A1c and fasting blood glucose were determined from blood samples taken at baseline and at the end of treatment from patients with DM treated with ivabradine and comparator drugs.
The data from the different studies were pooled when appropriate. Data relating to changes in the heart rate at rest and angina attack frequency were collected using similar methods in several studies; these data were pooled. However, different ETT procedures were used in the main efficacy studies. Because bicycle ETTs can yield smaller apparent treatment effects than treadmill ETTs, the ETT results and exercise heart rate variations across the studies could not be pooled. The data from 2 large studies, in which ivabradine was given as monotherapy and compared with the β blocker atenolol using treadmill ETTs (study 2) and with the dihydropyridine calcium channel blocker amlodipine using bicycle ETTs (study 4), are presented individually.
Pharmacokinetic analyses were performed in a subset of patients in all studies from whom blood samples were available. The maximum drug concentration and area under the curve for ivabradine and its active metabolite, S 18982, were determined for each patient, normalized to an ivabradine dose of 5 mg twice daily, and pooled for comparison of patients with and without DM.
All patients with DM with evaluable data were included in the analysis of blood concentrations of hemoglobin A1c and fasting glucose, and the data were pooled across studies. The changes from baseline with treatment were compared between ivabradine and the comparator drugs atenolol and amlodipine.
All analyses for the present study were performed post hoc and were not planned in the original study protocols. Statistical significance testing, when performed, was done using Student’s t test (unpaired) and should be considered exploratory.
Results
Patients with DM were approximately 18% of the total patient sample. Thus, the analyses of efficacy and heart rate changes were done using data from 535 patients with and 2,372 patients without DM treated with ivabradine. However, the number of patients varied for the different analyses, depending on data availability. For example, the pharmacokinetic analysis, for which only a subset of patients was tested, involved 788 patients treated with ivabradine (146 with DM). The baseline demographic and clinical characteristics of patients in the pooled sample used for the analyses of the efficacy and heart rate changes are listed in Table 2 . Of the ivabradine-treated patients, those with DM were slightly older, a greater proportion were women, and a greater proportion had relatively severe (Canadian Cardiovascular Society grade III) angina compared with the patients without DM. Far fewer patients were treated with comparator drugs than with ivabradine. Therefore, the statistical estimates of differences between the patients with and without DM in the comparator groups were less precise than those for ivabradine-treated patients.
| Characteristic | No DM | DM | ||||
|---|---|---|---|---|---|---|
| Ivabradine (n = 2372) | Atenolol (n = 352) | Amlodipine (n = 355) | Ivabradine (n = 535) | Atenolol (n = 83) | Amlodipine (n = 49) | |
| Mean age (years) | 59.9 ± 9.0 | 60.6 ± 8.8 | 59.7 ± 9.1 | 61.5 ± 8.2 | 61.2 ± 7.9 | 62.1 ± 7.4 |
| Gender | ||||||
| Male | 85% | 82% | 87% | 77% | 80% | 82% |
| Female | 16% | 19% | 13% | 23% | 21% | 18% |
| Coronary artery disease history | ||||||
| Myocardial infarction | 52% | 54% | 45% | 54% | 47% | 49% |
| Coronary artery bypass | 18% | 18% | 13% | 17% | 25% | 20% |
| Angioplasty | 16% | 18% | 11% | 20% | 23% | 14% |
| Anginal pain grade ⁎ | ||||||
| Grade I | 21% | 25% | 11% | 15% | 22% | 12% |
| Grade II | 66% | 66% | 73% | 65% | 65% | 67% |
| Grade III | 13% | 9% | 16% | 17% | 10% | 20% |
| Grade IV | 0 | 0 | 0 | 0.2% | 0 | 0 |
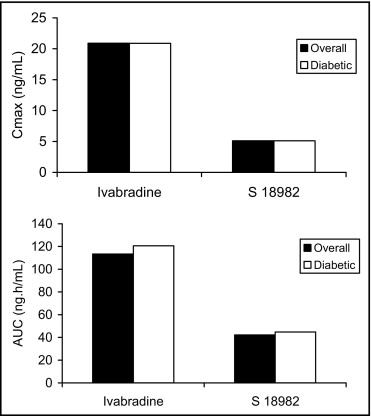
Ivabradine is eliminated with a half-life of 2 hours for the primary compound in plasma; the effective half-life for ivabradine and its active metabolite, S 18,982, is 11 hours. The mean plasma maximum drug concentration and area under the curve values for ivabradine and S 18982 were similar between the DM subset and the overall pharmacokinetic patient sample ( Figure 1 ), suggesting no obvious differences in the absorption, metabolism, and excretion of ivabradine in patients with DM.
The changes in the heart rate at rest after treatment with ivabradine and atenolol (all doses pooled) in patients with and without DM are summarized in Table 3 . At baseline, in the ivabradine group, the heart rate in those with DM was greater than in those without DM but the reduction in the heart rate with ivabradine treatment was similar in both groups. Similar results were seen in atenolol-treated patients.
| Diabetes Mellitus | Patients (n) | Heart Rate at Baseline (beats/min) | Change From Baseline (beats/min) |
|---|---|---|---|
| No | |||
| Ivabradine | 2231 | 71.9 ± 11.9 | −11.3 ± 11.1 (−15%) |
| Atenolol | 333 | 71.8 ± 12.2 | −12.1 ± 11.3 (−17%) |
| Yes | |||
| Ivabradine | 501 | 76.5 ± 12.8 | −11.6 ± 11.5 (−15%) |
| Atenolol | 80 | 76.7 ± 14.0 | −13.5 ± 12.6 (−18%) |
As noted, the ETT results for the 2 large efficacy studies (studies 2 and 4) of ivabradine as monotherapy are presented separately. In study 2 (treadmill ETTs), among those in the ivabradine group, the baseline values of the ETT parameters were all lower in the patients with DM relative to those without DM ( Table 4 ). However, the antianginal and anti-ischemic efficacy of ivabradine was proportionately similar in both groups. The results in the patients with DM were similar for ivabradine and atenolol ( Table 4 ). In study 4 (bicycle ETTs), all ETT parameters at baseline again were lower in those with DM compared with those without DM ( Table 5 ). Improvements in the ETT parameters with treatment were smaller in study 4 than in study 2 for all ETT parameters and for both ivabradine and the comparator drug, amlodipine. Improvements with treatment were similar in patients with and without DM for the parameters time to 1-mm ST segment depression and time to angina onset. Improvements in total exercise duration were smaller among those with DM than among those without DM in both the ivabradine and the amlodipine treatment groups; however, the results in the patients with DM were similar with ivabradine and amlodipine for all ETT parameters.
| Variable | Patients (n) | Baseline (s) | Change From Baseline (s) |
|---|---|---|---|
| Total exercise duration | |||
| No diabetes mellitus | |||
| Ivabradine, all doses | 475 | 602.9 ± 128.3 | 92.1 ± 124.0 (15%) |
| Atenolol 100 mg once daily | 224 | 573.6 ± 146.7 | 75.0 ± 132.4 (13%) |
| Diabetes mellitus | |||
| Ivabradine, all doses | 123 | 554.1 ± 150.5 | 77.9 ± 123.7 (14%) |
| Atenolol 100 mg once daily | 62 | 595.2 ± 134.8 | 92.6 ± 137.3 (16%) |
| Time to 1-mm ST-segment depression | |||
| No diabetes mellitus | |||
| Ivabradine, all doses | 471 | 541.0 ± 158.4 | 93.5 ± 139.2 (17%) |
| Atenolol 100 mg once daily | 224 | 507.9 ± 153.9 | 94.0 ± 143.7 (19%) |
| Diabetes mellitus | |||
| Ivabradine, all doses | 123 | 464.6 ± 166.6 | 88.5 ± 150.5 (19%) |
| Atenolol 100 mg once daily | 62 | 521.2 ± 164.3 | 101.3 ± 161.8 (19%) |
| Time to angina onset | |||
| No diabetes mellitus | |||
| Ivabradine, all doses | 474 | 482.1 ± 146.8 | 145.9 ± 150.8 (30%) |
| Atenolol 100 mg once daily | 223 | 454.5 ± 147.8 | 131.6 ± 152.4 (29%) |
| Diabetes mellitus | |||
| Ivabradine, all doses | 123 | 435.0 ± 144.3 | 129.1 ± 131.1 (30%) |
| Atenolol 100 mg once daily | 62 | 468.0 ± 135.2 | 148.1 ± 163.1 (32%) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


