TRITON-TIMI 38 showed that in patients with acute coronary syndrome undergoing percutaneous coronary intervention prasugrel decreased ischemic events compared to standard clopidogrel, but with more bleeding. The United States Food and Drug Administration and the European Medicines Agency approved prasugrel but provided contraindications in patients with previous stroke or transient ischemic attack and recommended limited use or reduced dose in patients ≥75 years old and weighing <60 kg. This defined 3 clinically relevant groups of patients for use of prasugrel at the studied dose regimen: group I (core clinical cohort), group II (noncore cohort), and group III (contraindicated). We assessed clinical outcomes of patients within these cohorts in the TRITON-TIMI 38 trial. Survival analysis methods were used to compare outcomes by treatment assignment (prasugrel vs clopidogrel) and by cohort (groups I and II or III). Patients in group I (n = 10,804, 79%) treated with prasugrel had a clinically significant and robust decrease in the primary end point of cardiovascular death, myocardial infarction, or stroke (8.3 vs 11.0%, hazard ratio [HR] 0.74, 95% confidence interval 0.66 to 0.84, p <0.0001), whereas patients in group II (n = 2149, 16%) had limited efficacy (15.3% vs 16.3%, HR 0.94, 0.76 to 1.18, p = 0.61, p for interaction = 0.07). For Thrombolysis In Myocardial Infarction major bleeding not related to coronary artery bypass grafting, there were tendencies to higher rates with prasugrel in group I (1.9% vs 1.5%, HR 1.24, 0.91 to 1.69, p = 0.17) and group II (4.1% vs 3.4%, HR 1.23, 0.77 to 1.97, p = 0.40); however, the absolute difference was greater for group II. The net clinical outcome (all-cause death/myocardial infarction/stroke/Thrombolysis In Myocardial Infarction major bleeding) in group I patients was highly favorable (10.2% vs 12.5%, HR 0.80, 0.71 to 0.89, p <0.0001) and neutral in group II (19.5% vs 19.7%, HR 0.98, 0.81 to 1.20, p for interaction = 0.07). Patients in group III (n = 518, 4%) did poorly with regard to efficacy and safety. In TRITON-TIMI 38 patients without previous stroke, <75 years old, and weighing >60 kg had substantial decreases in ischemic events with prasugrel compared to clopidogrel. Although relative bleeding excess exists in this population, absolute rates and differences in bleeding were attenuated. In conclusion, these data indicate that use of prasugrel in a core clinical cohort that has been defined by regulatory action will maximize the benefit of prasugrel and limit the risk of adverse outcomes.
Prasugrel is a third-generation thienopyridine that achieves higher levels of platelet inhibition more rapidly and consistently than standard- or high-dose clopidogrel. The TRial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet InhibitioN with Prasugrel (TRITON)–Thrombolysis In Myocardial Infarction (TIMI) 38 compared prasugrel to clopidogrel in patients with acute coronary syndromes (ACSs) and planned percutaneous coronary intervention. The trial showed a decrease in the primary composite end point of cardiovascular death, myocardial infarction (MI), and stroke over a period of 15 months but with more major and minor bleeding. Overall benefit/risk balance was considered favorable, and the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMEA) approved prasugrel for clinical use. Based on drug exposure modeling and analyses from TRITON-TIMI 38 of prespecified clinical features, 3 major subgroups of interest (weight <60 kg, >75 years old, and known history of stroke/transient ischemic attack [TIA]) were identified in which the net clinical outcome was neutral or favored clopidogrel. Unlike the overall cohort these groups had a neutral or unfavorable net outcome of all-cause death, MI, stroke, or TIMI major bleeding. For patients with previous stroke or TIA, prasugrel was considered contraindicated. For patients >75 years old the FDA indicated that prasugrel was generally not recommended but could be considered for use if additional high-risk factors for ischemic events such as previous MI were present. For patients <60 kg, in whom pharmacokinetic modeling suggests relative overexposure to the active metabolite of prasugrel, the FDA recommended consideration of a 5-mg maintenance dose. The EMEA approved prasugrel for use in patients with ACS undergoing percutaneous coronary intervention, indicating that the drug at standard dosing was generally not recommended in patients ≥75 years old and in patients weighing <60 kg. For older patients and those with low body weight, the EMEA recommended a 5-mg/day maintenance dose when prasugrel is used. Similar to the FDA, the EMEA also determined that prasugrel was contraindicated in patients with previous stroke or TIA. These characteristics allow for the division of the population into 3 regulatory-based subgroups with implications for use of the drug in clinical practice. Group I consists of patients who would be considered the “core” clinical population for treatment with full-dose prasugrel without limitation, i.e., patients without a known history of stroke or TIA, <75 years old, and weighing ≥60 kg. Group II is considered a “noncore” cohort for treatment with prasugrel, i.e., patients ≥75 years or weighing <60 kg but without known stroke or TIA. Group III is composed of patients for whom prasugrel is contraindicated, i.e., patients with known previous stroke or TIA. To provide caregivers information on what might be expected if prasugrel were administered in place of clopidogrel in patients with ACS undergoing percutaneous coronary intervention, we sought to assess outcomes of patients from these 3 defined groups (core, noncore, contraindicated) in the TRITON-TIMI 38 trial.
Methods
The design and results of the TRITON-TIMI 38 trial have been published previously. Thirteen thousand six hundred eight patients with moderate- to high-risk ACS with planned percutaneous coronary intervention were enrolled. Patients were treated with study medication (prasugrel or clopidogrel) for a median of 14.5 months. Patients were excluded if they had received a thienopyridine within 5 days, had active bleeding, or a bleeding diathesis. There were no limitations for enrollment by age, body weight, or kidney function. The primary efficacy end point was the composite of cardiovascular death, nonfatal MI, and nonfatal stroke. The key safety end point was noncoronary artery bypass grafting–related TIMI major bleeding.
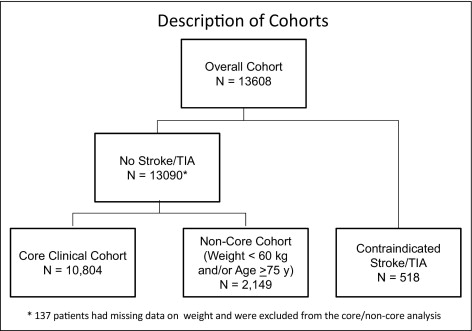
Cohorts were defined sequentially based on patient characteristics at enrollment. Subjects with a known history of stroke or TIA in whom prasugrel is contraindicated (group III) were removed from the overall population first regardless of age or weight. One hundred thirty-seven patients (1%) without stroke but with missing baseline weight data were excluded from the analysis because it could not be determined to which final cohort they should be assigned. The remaining subjects without previous stroke or TIA were categorized as group I or group II based on age and weight at the start of the trial. Survival analytic methods were used to compare outcomes by treatment assignment (prasugrel vs clopidogrel) and by whether or not subjects were included within the core cohort. Event rates are reported using Kaplan–Meier estimates at 450 days unless stated otherwise. Comparisons are expressed as hazard ratios (HRs) and 95% confidence intervals including the entire duration of follow-up. Testing for interaction between the efficacy/safety of prasugrel compared to clopidogrel by cohort status was performed by constructing a Cox proportional hazards model using terms for the main effect and the interaction. For all analyses 2-sided p values <0.05 were considered statistically significant. Early events are reported using Kaplan–Meier estimates at 30 days and late events using landmark analyses excluding nonfatal events before 30 days. All analyses were performed using STATA/SE 9.2 (STATA Corporation, College Station, Texas).
The sponsors of TRITON-TIMI 38 trial (Daiichi Sankyo Company, Limited, Parsippany, New Jersy and Eli Lilly and Company, Indianapolis, Indiana) supported the design and implementation of the main trial from which these results are obtained. All analyses were performed by the TIMI Study Group using an independent copy of the complete clinical trial database. The authors wrote all drafts of the report and take responsibility for its content. The sponsors had the opportunity to review and comment on this report but had no editorial authority.
Results
Of 13,608 subjects from the TRITON-TIMI 38 trial ( Figure 1 ) , the population included 10,804 subjects (79%) in group I (core cohort), 2,149 (16%) in group II (noncore), and 518 (4%) in group III (contraindicated). As defined subjects in group I were younger, heavier, and had no history of stroke or TIA ( Table 1 ). Group I subjects were also more likely to have ST-segment elevation MI as their presenting syndrome, were more often men, more often smokers, and more frequently received glycoprotein IIb/IIIa inhibitors, β blockers, and statins. Group I subjects were less likely to have a history of hypertension, diabetes, previous MI, chronic kidney disease, or multivessel percutaneous coronary intervention. Baseline characteristics of subjects in group I randomized to prasugrel or clopidogrel were well matched. In total 78% of group II subjects were ≥75 years old and 30% weighed <60 kg. Aside from the uniform prevalence of stroke or TIA, patients in group III more frequently were women, hypertensive, had more previous MI, and more frequent diabetes than the core cohort. Stent types and aspirin use were similar between groups. There was no difference in the proportion of patients in groups who were randomized to receive prasugrel ( Table 1 ).
| Group I | Group II | Group III | |
|---|---|---|---|
| (n = 10,804) | (n = 2,149) | (n = 518) | |
| Non–ST-segment elevation myocardial infarction/unstable angina pectoris | 73% | 76% | 78% |
| Age ≥75 years | 0% | 78% | 24% |
| Weight <60 kg | 0% | 30% | 6% |
| Previous stroke/transient ischemic attack | 0% | 0% | 100% |
| Men | 79% | 50% | 67% |
| White race | 93% | 93% | 89% |
| Region | |||
| North America | 32% | 29% | 35% |
| South America | 4% | 5% | 4% |
| Western Europe | 25% | 33% | 22% |
| Eastern Europe | 25% | 21% | 22% |
| Africa/Asia/Middle East | 14% | 11% | 17% |
| Hypertension | 62% | 71% | 88% |
| Hypercholesterolemia | 56% | 51% | 65% |
| Diabetes mellitus | 22% | 23% | 38% |
| Tobacco use | 43% | 18% | 26% |
| Previous myocardial infarction | 17% | 21% | 33% |
| Previous coronary bypass | 7% | 11% | 14% |
| Creatinine clearance <60 ml/min | 4% | 43% | 21% |
| Bare-metal stent only | 48% | 49% | 42% |
| Drug-eluting stent | 47% | 45% | 51% |
| Multivessel coronary intervention | 14% | 16% | 20% |
| Glycoprotein IIb/IIIa inhibitor | 56% | 49% | 47% |
| Angiotensin-converting enzyme/angiotensin receptor blocker | 75% | 77% | 84% |
| β Blocker | 89% | 86% | 88% |
| Statin | 93% | 89% | 90% |
| Calcium channel blocker | 16% | 23% | 29% |
| Aspirin | 100% | 99% | 99% |
| Randomized to prasugrel | 50% | 49% | 51% |
Patients in group I treated with prasugrel compared to clopidogrel exhibited a robust decrease in the primary end point (8.3% vs 11.0%, HR 0.74, 0.66 to 0.84, p <0.0001; Figure 2 ) . This benefit was consistent for MI alone and for the combination of cardiovascular death and MI ( Supplementary Table 1 ). Compared to group I the primary efficacy end point occurred more frequently in group II regardless of treatment assignment. In group II there was less efficacy with nearly neutral outcomes (15.3% vs 16.3%, HR 0.94, 0.76 to 1.18, p = 0.61). The interaction (p for interaction = 0.07) between the benefit of prasugrel and being in group I versus group II approached statistical significance. In the 2 groups there was no difference between prasugrel and clopidogrel in the incidence of stroke. In contrast to other outcomes, treatment with prasugrel was associated with a similar decrease in the incidence of stent thrombosis in group I (1.0% vs 2.3%, HR 0.44, 0.31 to 0.62, p <0.0001) and in group II (1.5% vs 2.5%, HR 0.63, 0.32 to 1.22, p = 0.17, p for interaction = 0.36) as seen in Figure 2 .
This pattern of efficacy outcomes was consistent across multiple subgroups of group I ( Figure 3 ) . In group II ( Figure 3 ) the pattern of limited efficacy was largely consistent across subpopulations with the notable exception of subjects with diabetes in whom a substantial benefit was noted with a significant interaction between treatment and outcome (p for interaction = 0.01).




