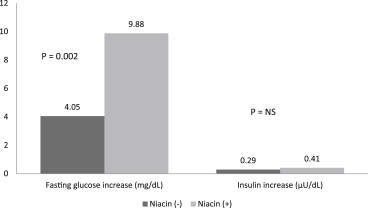
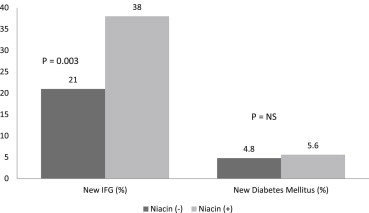
Although the effect of niacin on the glucose levels in subjects with diabetes mellitus has been investigated, niacin’s effects on the glucose levels and atherosclerosis in subjects with normal glucose levels have not been well established. We examined the effect of niacin on the glucose levels, coronary stenosis progression using quantitative coronary angiography, and clinical events in 407 subjects who had a baseline glucose level <100 mg/dl and were enrolled in the Familial Atherosclerosis Treatment Study (FATS), HDL-Atherosclerosis Treatment Study (HATS), Armed Forces Regression Study (AFREGS), or Carotid Plaque Composition by MRI during lipid-lowering (CPC) study testing active niacin therapy. Although the fasting glucose levels increased significantly within 3 years in both subjects treated with niacin (from 85.6 ± 9.5 to 95.5 ± 19.7 mg/dl, p <0.001) and without niacin (from 85.2 ± 9.6 to 90 ± 17.9 mg/dl, p = 0.009), those treated with niacin had a significantly larger increase in glucose levels than those not taking niacin (9.88 vs 4.05 mg/dl, p = 0.002). Overall, 29% of subjects developed impaired fasting glucose within 3 years. Incident impaired fasting glucose was significantly more likely to be observed in subjects treated with niacin than in those who were not. However, the frequency of new-onset diabetes mellitus did not differ significantly between the 2 groups (5.6% vs 4.8%, p = 0.5). Niacin-treated subjects compared to untreated subjects had significantly less change in mean coronary stenosis (0.1 ± 0.3% vs 2 ± 12%, p <0.0001) and less major cardiovascular events (8% vs 21%, p = 0.001). In conclusion, the use of niacin for 3 years in subjects with normal baseline glucose levels was associated with an increase in blood glucose levels and the risk of developing impaired fasting glucose, but not diabetes mellitus, and was associated with a significantly reduced incidence of coronary stenosis progression and major cardiovascular events.
Niacin has been widely used to treat dyslipidemia with elevated triglycerides and low levels of high-density lipoprotein (HDL) cholesterol. Multiple randomized, placebo-controlled trials, including the Coronary Drug Project (CDP), have demonstrated the ability of niacin to reduce the progression of atherosclerosis and lower the incidence of cardiovascular events in patients with established vascular disease. However, a noted side effect of niacin therapy has been an observed increase in serum glucose levels. Although several randomized control trials have evaluated the glucose effects of niacin in those with diabetes mellitus (DM), insufficient data are available to clearly establish the effect of niacin on glucose levels, incident DM, and cardiovascular disease in a population of subjects with baseline normal glucose levels. We, therefore, performed the present combined analysis to examine the effects of niacin therapy on glucose levels, coronary stenosis progression, and clinical events in subjects with normal baseline fasting glucose levels using combined data from 4 randomized lipid trials testing active niacin treatment, including the Familial Atherosclerosis Treatment Study (FATS), HDL-Atherosclerosis Treatment Study (HATS), Armed Forces Regression Study (AFREGS), and Carotid Plaque Composition by MRI [magnetic resonance imaging] during lipid-lowering (CPC) study.
Methods
A total of 407 subjects with established vascular disease without a diagnosis of DM who had participated in the FATS, HATS, AFREGS, and CPC study and had been treated with or without niacin and had a baseline fasting glucose level <100 mg/dl were included in the present analysis. The full protocols of these studies have been previously published. In brief, the FATS enrolled 146 men from 1984 to 1987 who had elevated apolipoprotein B levels ≥125 mg/dl, a family history of coronary heart disease, and documented coronary atherosclerosis on an angiogram. The subjects were randomized to lovastatin (20 mg twice daily) and colestipol (10 g 3 times daily), immediate release niacin (1 g 4 times daily) and colestipol (10 g 3 times daily), or placebo. The HATS enrolled 160 subjects with a history of coronary artery disease, documented coronary atherosclerosis on the angiogram, and low HDL cholesterol levels (<35 mg/dl in men, <40 mg/dl in women). The subjects were randomized to slow-release niacin (2 to 4 g/day) plus simvastatin (10 to 20 mg/day); antioxidants; slow-release niacin (2 to 4 g/day), simvastatin (10 to 20 mg/day) plus antioxidants; or all placebo. The AFREGS enrolled 143 military retirees with documented coronary atherosclerosis by angiogram and HDL cholesterol <40 mg/dl and randomized them to immediate-release niacin (3 g/day), cholestyramine (16 g/day) plus gemfibrozil (600 mg twice daily), or placebo. The CPC study randomized 123 subjects with coronary or carotid artery disease, apolipoprotein B >120 mg/dl, and <1 year of lipid therapy to atorvastatin (10 to 80 mg/day); atorvastatin plus extended-release niacin (2 g/day); or atorvastatin and extended release niacin plus colesevelam (3.8 g/day).
The fasting glucose levels were measured at baseline and throughout each study for 3 years per protocol. A diagnosis of new-onset impaired fasting glucose (IFG) was defined as a glucose level of 100 to 125 mg/dl on ≥2 measurements during treatment, and new-onset DM was defined as fasting glucose levels of ≥126 mg/dl on ≥2 measurements during treatment. The on-treatment glucose levels and frequencies of new-onset IFG and DM were compared between the subjects treated with and without niacin. Of the 4 studies, the FATS, HATS, and AFREGS had the same clinical end points, including the percentage of change in mean proximal coronary stenosis, as measured by quantitative coronary angiography, and major cardiovascular events, including death, myocardial infarction, stroke, and revascularization because of worsening ischemia. A total of 342 subjects in these 3 trials had a baseline glucose level <100 mg/dl and were included in the comparison. Continuous variables are reported as the mean ± SD. Comparisons were made using 2-sided t tests, chi-square tests, and Wilcoxon-Mann-Whitney tests, as appropriate. p Values <0.05 were considered significant.
Results
Of the 407 subjects in the 4 studies with a baseline glucose level <100 mg/dl, 197 received active treatment with niacin and 210 were not treated with niacin. The subjects’ mean age was 58.7 ± 9.0 years, their body mass index was 27.4 ± 4.1, and 90.5% were men ( Table 1 ). The percentage of men in the niacin group was lower than in the group without niacin (85% vs 96%, p = 0.001).
| Variable | Niacin Treatment | p Value ∗ | |
|---|---|---|---|
| No (n = 210) | Yes (n = 197) | ||
| Age (yrs) | 59.1 ± 8.7 | 58.3 ± 9.2 | 0.39 |
| Men | 201 (96%) | 168 (85%) | 0.001 |
| Body mass index (kg/m 2 ) | 27.2 ± 4.0 | 27.6 ± 4.1 | 0.44 |
| Metabolic syndrome | 85 (40%) | 79 (40%) | 0.85 |
| Baseline high-density lipoprotein cholesterol (mg/dl) | 35.8 ± 8.4 | 35.6 ± 7.8 | 0.77 |
| Baseline low-density lipoprotein cholesterol (mg/dl) | 150.6 ± 51.9 | 144.6 ± 45.8 | 0.21 |
| Baseline triglycerides (mg/dl) | 190.2 ±115.2 | 194.3 ± 126.6 | 0.73 |
∗ p Values for continuous data were obtained by t tests (or Mann-Whitney U tests when appropriate); for categorical data, chi-square tests were used.
The fasting glucose levels increased significantly during the 3-year period in the subjects treated with niacin (from 85.6 ± 9.5 to 95.5 ± 19.7 mg/dl, p <0.001) and those treated without niacin (from 85.2 ± 9.6 to 90.0 ± 17.9 mg/dl, p = 0.009; Figure 1 ). The subjects treated with niacin had a significantly larger increase in glucose levels than those not taking niacin (9.88 vs 4.05 mg/dl, p = 0.002). Although a difference was seen in the amount of change in the glucose levels, the insulin levels increased nonsignificantly in the subjects treated without niacin (from 17.2 ± 13.2 to 17.6 ± 13.3 μU/dl, p = 0.77) and in those treated with niacin (from 19.9 ± 16.4 to 21.3 ± 16.0 μU/dl, p = 0.86). Furthermore, no significant difference was seen in the change in insulin levels between the niacin-treated and untreated groups (0.41 vs 0.29 μU/dl, p = 0.36). The glucose increase was not associated with the type or dosage of niacin used. Overall, 122 of 407 (29%) of subjects developed IFG during the 3-year period. IFG was significantly more likely to be seen in subjects treated with niacin than in those without niacin treatment (38% [78 of 197] vs 21% [44 of 210], p = 0.003; Figure 2 ). A nonsignificant greater number of incident DM was found in the niacin group (5.6% [11 of 197] vs 4.8% [10 of 210]; p = 0.5).