Congenitally corrected transposition of the great arteries (CCTGA) is associated with tricuspid regurgitation (TR), which has been postulated to arise from the effect of ventricular septal position on the attachments of the tricuspid valve. This study was performed to determine the effect of left ventricular (LV) pressure on right ventricular (RV) and LV geometry and the degree of TR. Serial echocardiograms were reviewed from, 30 patients with CCTGA who underwent pulmonary artery banding to train the morphologic left ventricle (n = 14) or left ventricle–to–pulmonary artery conduit placement and ventricular septal defect closure in conjunction with physiologic repair (n = 16). The degree of TR, the LV/RV pressure ratio, RV and LV sphericity indexes, and tricuspid valve tethering distance and coaptation length were analyzed. After pulmonary artery banding, an increase in LV systolic pressure to ≥2/3 systemic resulted in a decrease in TR from severe to moderate (p = 0.02). The percentage of patients with severe TR decreased from 64% to 18% (p = 0.06). The RV sphericity index decreased (p = 0.05), and the LV sphericity index increased (p = 0.02). After left ventricle–to–pulmonary artery conduit placement, a decrease in LV pressure to ≤1/2 systemic resulted in an increase in TR from none to mild (p = 0.003). In conclusion, these data indicate that LV pressure in patients with CCTGA affects the degree of TR and that septal shift caused by changes in LV and RV pressure is an important mechanism.
Congenitally corrected transposition of the great arteries (CCTGA) results in the morphologic right ventricle developing as the systemic ventricle, while the morphologic left ventricle develops as the subpulmonary ventricle. Tricuspid regurgitation (TR) is a major factor associated with increased morbidity and mortality in patients with CCTGA, and 20% to 50% of patients with this condition have been demonstrated to have at least moderate TR. The causes of TR in CCTGA are not well understood and may be multifactorial. The absence of substantial TR in many patients with CCTGA, even those with morphologically abnormal tricuspid valves, makes morphologic abnormalities unlikely to be the sole cause. It has been observed that TR increases after conventional or physiologic repairs, in which the tricuspid valve is left in the systemic circulation, and TR decreases after procedures that increase left ventricular (LV) pressure, such as pulmonary artery (PA) banding. This study was undertaken to test the hypothesis that increasing the LV/right ventricular (RV) pressure ratio with PA banding results in a less spherical right ventricle, improved tricuspid valve coaptation, and decreased TR. Alternatively, decreasing the LV/RV pressure ratio after physiologic repair results in a more spherical right ventricle, increased tethering of tricuspid valve leaflets, diminished tricuspid valve coaptation, and an increase in the degree of TR.
Methods
Review of the University of Michigan Health System’s Division of Pediatric Cardiology echocardiographic database identified all patients with CCTGA who underwent surgical interventions that were expected to alter the LV/RV pressure ratio while leaving the right ventricle as the systemic ventricle. Patients requiring single-ventricle palliation were excluded. We examined 2 groups of patients. Those in group 1 underwent PA banding to increase LV pressure in preparation for eventual double-switch procedures (anatomic repair; n = 14). Those in group 2 underwent procedures that would decrease their LV pressure, either physiologic repair involving ventricular septal defect closure with left ventricle–to–pulmonary artery conduit placement or the replacement of a stenotic left ventricle–to–pulmonary artery conduit (n = 16). Medical records were retrospectively reviewed to determine surgical procedures and dates of echocardiography. This study was approved by the University of Michigan Institutional Review Board, with a waiver of informed consent.
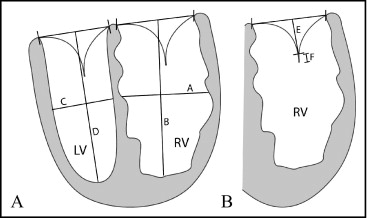
In the PA banding patients (group 1), the preoperative echocardiogram and the echocardiogram obtained just before the next surgical procedure (most commonly anatomic repair) were evaluated. Because there is often an increase in the PA banding gradient over time because of growth, the echocardiogram just before the next surgical procedure was chosen to ensure that the maximum increase in LV pressure was achieved. For the physiologic repair patients (group 2), the preoperative and first adequate postoperative echocardiograms were evaluated. The echocardiogram immediately after operative repair was chosen to minimize the increase in LV pressure that can occur from progressive left ventricle–to–pulmonary artery conduit stenosis. Measurements were made of RV and tricuspid valve geometry. All measurements were made from the apical 4-chamber view. The tethering distance and length of coaptation of the tricuspid valve, RV sphericity index, and LV sphericity index were measured as demonstrated in Figure 1 . The tethering distance of the tricuspid valve was measured as the shortest distance to the coaptation point of the tricuspid valve from a line through the tricuspid valve annulus, and the coaptation length was measured as the length of overlap of the tricuspid valve leaflets. Tethering distance and coaptation length were indexed to the patient’s body surface area. TR was graded on a 4-point scale as none or trivial, mild, moderate, or severe (labeled 0 to 3 in figures) on the basis of the size of the jet, dilatation of the left atrium or right ventricle, and reversal of flow in the pulmonary veins. Each measurement was performed and verified by a single observer who was blinded to the treatment and results.
The subpulmonary, morphologic LV systolic pressure was measured at cardiac catheterization (12 patients preoperatively, 8 patients postoperatively) or estimated by echocardiography (18 patients preoperatively, 22 patients postoperatively) using maximal mitral regurgitation gradient added to an estimated right atrial pressure of 5 mm Hg, maximal pulmonary outflow gradient added to an estimated pulmonary artery pressure of 20 mm Hg, or maximal ventricular septal defect gradient subtracted from systolic blood pressure. The LV/RV systolic pressure ratio was estimated by dividing the estimated LV systolic pressure by the systolic blood pressure, because no patient had an RV outflow tract obstruction.
The degree of TR was compared preoperatively and postoperatively using Wilcoxon’s rank test. Pre- and postoperative measures of RV and tricuspid valve geometry were compared using a 2-tailed, paired Student’s t test or McNemar’s test, as appropriate. Patients in the PA banding group who did not have increases in their LV/RV pressure ratios to ≥2/3 of RV pressure were excluded from statistical analysis. Patients in the physiologic repair group who did not have decreases in their LV/RV pressure ratios to ≤1/2 systemic pressure were also excluded from statistical analysis.
Results
Fourteen patients with CCTGA underwent PA banding placement for retraining of the morphologic left ventricle (group 1). The median age in this group was 1.1 years (range 0 to 12.1). Associated lesions included small ventricular septal defects; mild pulmonary stenosis; thickened, apically displaced tricuspid valves; and complete heart block, with 2 patients requiring pacemakers preoperatively and 2 additional patients requiring 1 postoperatively ( Table 1 ). The median time interval between measurements was 3.6 months (range 0.1 to 48.6). Eleven of the 14 patients (79%) had increases in their LV pressure to ≥2/3 of RV systolic pressure after PA banding placement. Among those 11 patients, TR decreased from a median of severe preoperatively (range none or mild to severe) to moderate postoperatively (range none or mild to severe) (p = 0.05; Figure 2 ). The percentage of patients with severe TR decreased from 64% to 18% (p = 0.06). In addition, the RV sphericity index decreased from a mean of 0.85 ± 0.22 to 0.69 ± 0.18 (p = 0.05), and the LV sphericity index increased from a mean of 0.37 ± 0.09 to 0.48 ± 0.09 (p = 0.02). Tricuspid valve tethering distance and coaptation length were not different between pre- and postoperative measurements ( Table 2 ).
| Patient | Gender | Age | Other Defects | Previous Surgeries | Echocardiography Interval (days) |
|---|---|---|---|---|---|
| Group 1 | |||||
| 1 | M | 2 wk | Small VSD, ASD, CHB | 0 | 398 |
| 2 | M | 2 mo | Ebsteinoid TV | 0 | 7 |
| 3 | M | 3 mo | CoA, Ebsteinoid TV | 0 | 11 |
| 4 | M | 4 mo | Dextrocardia, small VSD, Ebsteinoid TV | 0 | 4 |
| 5 | F | 5 mo | CoA | CoA repair, pacemaker | 5 |
| 6 | M | 7 mo | ASD, small VSD, mild PS | 0 | 549 |
| 7 | F | 12 mo | None | 0 | 156 |
| 8 | F | 14 mo | Dextrocardia, mild PS, WPW | 0 | 1478 |
| 9 | M | 16 mo | VSD, CHB | VSD repair, pacemaker | 5 |
| 10 | F | 4 yrs | Small VSD | 0 | 124 |
| 11 | M | 5 yrs | Ebsteinoid TV | 0 | 705 |
| 12 | M | 6 yrs | VSD, mild PS, CHB | VSD repair, pacemaker | 108 |
| 13 | M | 12 yrs | Small VSD, Ebsteinoid TV | 0 | 5 |
| 14 | F | 12 yrs | None | 0 | 111 |
| Group 2 | |||||
| 1 | F | 4 mo | ASD, VSD, mild PS, CHB | 0 | 60 |
| 2 | M | 4 mo | VSD | 0 | 54 |
| 3 | M | 6 mo | VSD, pulmonary atresia | BTS | 11 |
| 4 | M | 22 mo | VSD, pulmonary atresia | BTS | 10 |
| 5 | M | 2 yrs | VSD, PS | 0 | 80 |
| 6 | F | 2 yrs | Dextrocardia, ASD, VSD, PS | 0 | 5 |
| 7 | M | 3 yrs | VSD, PS | BTS | 4 |
| 8 | F | 3 yrs | VSD, PS | 0 | 15 |
| 9 | F | 4 yrs | ASD, VSD, PS | BTS | 89 |
| 10 | M | 5 yrs | VSD, PS | 0 | 16 |
| 11 | F | 8 yrs | ASD, VSD, PS | BTS, PDA ligation | 701 |
| 12 | M | 9 yrs | VSD, pulmonary atresia | BTS, VSD repair, LV conduit | 92 |
| 13 | F | 11 yrs | Dextrocardia, VSD, pulmonary atresia | BTS, VSD repair, LV conduit | 4 |
| 14 | M | 17 yrs | ASD, VSD, PS, Ebsteinoid TV | VSD repair, LV conduit | 182 |
| 15 | M | 17 yrs | Dextrocardia, ASD, VSD, PS | WS, VSD repair, LV conduit | 94 |
| 16 | F | 40 yrs | VSD, PS | VSD repair, LV conduit | 160 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


