Chapter 12 Effects of high altitude
ALTITUDE AND OXYGEN TRANSPORT
Effects of altitude on plasma oxygen uptake
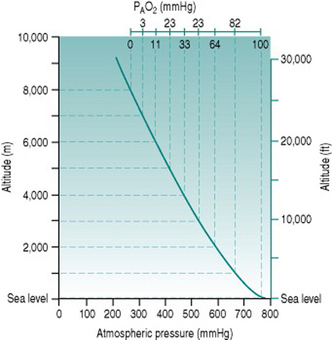
The standard atmospheric pressure of 760 mmHg (101 kPa) at sea level reflects the weight exerted by the gas molecules that make up the air, under gravitational force. As one ascends from sea level, the air becomes progressively less compressed and so the constituent gas molecules become less tightly packed. In consequence, a given volume of inspired air contains fewer molecules of all gases, including oxygen. The relationship between altitude and atmospheric pressure is not a strictly linear one because the air volume increases in 3 dimensions but, in rough terms, pressure falls by around 100 mmHg for every 1000 m (3300 ft) of ascent up to 3000 m (10 000 ft) (Fig. 12.1).
The absolute change in oxygen availability imposed by a given ascent can be calculated easily. Since oxygen represents 21% of normal air, the partial pressure of oxygen (PO2) at sea level is (21%.760) or 160 mmHg. Once inspired, the air becomes saturated with water vapour (partial pressure 47 mmHg) so that the total gas pressure is reduced to 713 mmHg and PO2 falls to (21%.713) or 150 mmHg. In the alveoli, the oxygen is diluted further by approximately 50 mmHg, due primarily to the presence of carbon dioxide, resulting in a local PO2 (PAO2) of around 100 mmHg: at equilibrium with the plasma, arterial PO2 (PaO2) is, therefore, usually also around 100 mmHg.
Effects of altitude on oxygen carriage
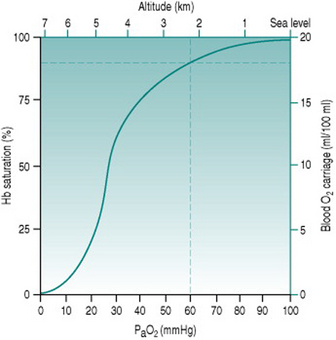
Because of the sigmoid shape of the haemoglobin dissociation curve, the falls in PaO2 associated with acute exposure to altitudes up to around 2000 m (6700 ft) cause only a slight reduction in haemoglobin saturation (Fig. 12.2) and so do not reduce oxygen delivery at rest. During exercise, however, the combination of reduced binding and reduced pulmonary capillary transit time leads to a greater degree of desaturation that is proportional both to altitude and to cardiac output. Thus, the threshold altitude for oxygen limitation of maximum exercise in sedentary individuals is typically around 1500 m (5000 ft), but, in trained athletes with substantially greater cardiac outputs, maximum work capacity begins to fall at much lower altitudes (Johnson et al 1994). In the only Summer Olympics held at a significant altitude, in Mexico City in 1968, winning times for all track events longer than 800 m were well above the existing records.
COMPENSATION FOR HYPOXIA
Respiratory stimulation
The reduced oxygen carriage associated with moderate altitude results in more rapid fatigue during exercise, but no respiratory compensation occurs because ventilation is still driven by the central chemoreceptors. These respond to rises in local proton concentration (that is, reduced pH) secondary to arterial carbon dioxide diffusing into the hindbrain, but are insensitive to hypoxia. The peripheral chemoreceptors responsible for monitoring arterial oxygen status are triggered only when PaO2 falls to around 60 mmHg which, as we saw earlier (p. 145), corresponds to an approximate altitude of 2300 m or 7600 ft. At or above this height, chemoreceptor stimulation initiates increased minute ventilation the magnitude of which depends both on the initial PaO2 and on whether increased ventilation is able to restore PaO2 to a value above 60 mmHg.
Haematological changes
Over the first few days of exposure to hypoxia, haematological changes increase the capacity of the red blood cell pool to deliver oxygen. The alkalosis produced by hypocapnia increases red cell expression of 2,3-diphosphglycerate (DPG), which shifts the haemoglobin dissociation curve to the right and increases oxygen unloading in the systemic capillaries (Fig. 12.3). Simultaneously, erythropoietin release from the renal medulla in response to hypoxia stimulates red cell production, so that haematocrit rises progressively over the ensuing several weeks.
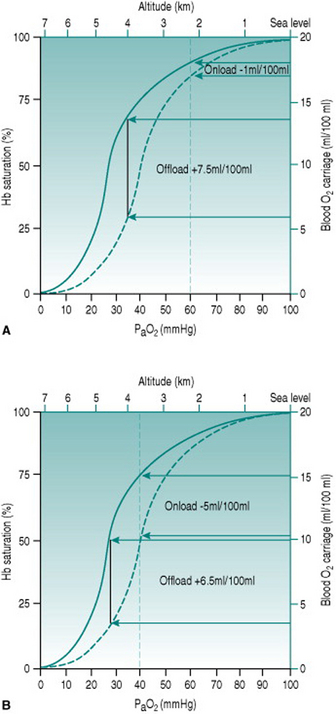
Figure 12.3 Effect of altitude on the efficiency of DPG expression as an aid to oxygen delivery. Note that the values for PaO2 represent those occurring at the respective altitudes before any compensation. (a) At altitude 2200 m (PaO2 60 mmHg) there is a doubling of oxygen offloading, while onloading is virtually unaffected. The net tissue gain is 6.5 ml oxygen/100 ml blood. (b) At altitude 3500 m (PaO2 40 mmHg), by contrast, the improved offloading is almost obviated by reduced onloading. The net tissue gain now is only 1.5 ml oxygen/100 ml blood.