Fetal supraventricular tachycardia (SVT) and atrial flutter (AF) can be associated with significant morbidity and mortality. Digoxin is often used as first-line therapy but can be ineffective and is poorly transferred to the fetus in the presence of fetal hydrops. As an alternative to digoxin monotherapy, we have been using sotalol at presentation in fetuses with SVT or AF with, or at risk of, developing hydrops to attempt to achieve more rapid control of the arrhythmia. The present study was a retrospective review of the clinical, echocardiographic, and electrocardiographic data from all pregnancies with fetal tachycardia diagnosed and managed at a single center from 2004 to 2008. Of 29 affected pregnancies, 21 (16 SVT and 5 AF) were treated with sotalol at presentation, with or without concurrent administration of digoxin. Of the 21, 11 (6 SVT and 5 AF) had resolution of the tachycardia within 5 days (median 1). Six others showed some response (less frequent tachycardia, rate slowing, resolution of hydrops) without complete conversion. In 1 fetus with a slow response, the mother chose pregnancy termination. The 5 survivors with a slow response were all difficult to treat postnatally, including 1 requiring radiofrequency ablation as a neonate. One fetus developed blocked atrial extrasystoles after 1 dose of sotalol and was prematurely delivered for fetal bradycardia. Three grossly hydropic fetuses with SVT showed no response and died within 1 to 3 days of treatment. In conclusion, transplacental sotalol, alone or combined with digoxin, is effective for the treatment of fetal SVT and AF, with an 85% complete or partial response rate in our series.
Sotalol, a safe and effective antiarrhythmic in pediatric and adult patients, is also useful in fetuses. Sotalol has been used as a second-line agent to treat fetal supraventricular tachycardia (SVT) with mixed results. In 2000, Oudijk et al reported discouraging results with sotalol in the treatment of fetal SVT because of the high fetal mortality associated with treatment. However, 3 of the 4 nonsurviving fetuses were grossly hydropic at presentation, a factor that likely contributed to their death. The same group subsequently documented good transplacental pharmacokinetics for sotalol and suggested, despite their earlier experience, that sotalol is still a reasonable choice for second-line fetal SVT therapy. Additionally, in studies of small cohorts of patients, sotalol appeared to have reasonable success in treating both fetal atrial flutter and long ventricular-atrial (VA) SVT, 2 conditions for which digoxin alone is generally ineffective. These encouraging studies suggested that sotalol might be a good first-line medication for the treatment of these fetal arrhythmias. As a step toward determining the efficacy of sotalol as first-line therapy in the treatment of fetal SVTs, we retrospectively reviewed our experience managing fetal SVT and atrial flutter (AF) in a single center in which sotalol as first-line therapy was the preferred approach.
Methods
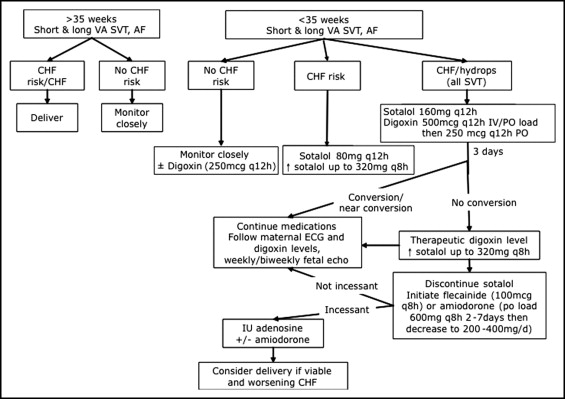
This was a descriptive retrospective study of all consecutively encountered pregnancies with fetal SVT evaluated and managed in our combined fetal cardiovascular program at the University of California, San Francisco, and University of California, Davis, from July 2004 to July 2008. The human subjects committees at both institutions approved the present study. Fetuses referred for tachycardia (>180 beats/min), who were found to have ventricular tachycardias, characterized by AV dissociation with ventricular rates in excess of the atrial rates, were excluded. The treatment protocol used by our fetal cardiovascular program is outlined in Figure 1 . After a diagnosis of fetal SVT or AF, and before the initiation of sotalol, the pregnant mothers underwent a baseline electrocardiogram (ECG) with or without an echocardiogram and an adult cardiology consultation. Contraindications to sotalol administration included a maternal history of cardiac disease with dysfunction, maternal bradycardia, and/or prolonged QTc. QTc-prolonging medications were discontinued, including antiemetics. The mothers were admitted to a nontelemetry bed on the obstetrical ward and had continuous fetal heart rate monitoring (or intermittent fetal heart rate monitoring 2 to 4 times daily, if pre-viable or if continuous monitoring was not feasible). The mothers were kept under observation during the initiation of sotalol or sotalol and digoxin therapy (digoxin added either at the treating physician’s discretion or if it was believed that the β blockade effect of sotalol would be detrimental in the context of overt fetal ventricular dysfunction). The dose of sotalol was increased after 48 hours if there was no or a minimal change in the frequency of SVT events. After initiation of sotalol (and after each dose adjustment), maternal ECGs were performed to assess the QTc intervals through the first 5 doses to exclude significant prolongation of maternal QTc. Serum electrolytes were monitored daily during the initiation and adjustment of sotalol, and potassium and magnesium supplementation were given orally as needed to maintain normal levels. Fetal echocardiography was repeated if a change occurred in the baseline rhythm. Routine biophysical profiles were not performed; however, obstetric ultrasound scans or echocardiograms were done daily or every other day for evaluation of effusions while the fetuses were in tachycardia.
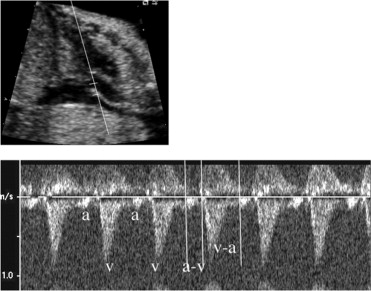
The maternal medical records were reviewed to document the gestational age at the diagnosis of fetal SVT or AF, treatment course, time to response, and any other pertinent pregnancy history, including maternal side effects. The postnatal medical records of the infants were reviewed to assess for postnatal arrhythmias, to confirm the tachycardia mechanism, the treatment course, the response to treatment, and the presence or absence of structural heart disease. Fetal echocardiograms were retrospectively reviewed by 2 experienced fetal and pediatric cardiologists to evaluate the mechanism of the tachycardia, to assess for hydrops, and to evaluate for structural heart disease. SVT and AF were diagnosed using standard M-mode and Doppler techniques. The mechanism of SVT in the fetus was defined according to Doppler-derived atrial and ventricular flow onset intervals, as previously described by Fouron et al ( Figure 2 ). Hydrops was defined as the presence of ≥2 of the following: ascites, pericardial effusion, pleural effusions, and skin edema. Pregnancies were defined as at risk of congestive heart failure using the observations of Naheed et al and included a diagnosis of SVT or AF that presented before 32 weeks gestation, incessant SVT (>50% of the examination), and the presence of structural heart disease.
An electrophysiologist reviewed all maternal ECGs performed before and after treatment onset, from which the maternal heart rates and QTc intervals were recorded. Infant ECGs, Holter monitor reports, and electrophysiologic studies were also reviewed to confirm the mechanism of tachyarrhythmia and evaluate the response to treatment.
Descriptive statistics, including the mean, median, and range, are reported. The gestational age at diagnosis, tachycardia mechanism and rate, and the presence or absence of hydrops were compared among rapid responders, partial or slow responders, and nonresponders to sotalol.
Results
A total of 29 pregnancies presented to our fetal cardiovascular program from July 2004 to July 2008 with fetal tachycardia, none of which had received previous treatment. Of these 29, 21 were treated with sotalol as per the described protocol, and the remainder were either treated with digoxin alone or were managed expectantly owing to intermittent nature of the tachycardia or diagnosis near term. In 4 nonhydropic fetuses, sotalol and digoxin were used at onset of treatment at the discretion of the involved fetal cardiologist, thus not fully conforming to the outlined protocol. Of the 21 treated with sotalol or sotalol and digoxin together, the gestational age at diagnosis of the fetal tachycardia ranged from 17 to 35 weeks (median 25). The mechanisms of tachycardia among treated patients included AF in 5 and SVT in 16. Of those with SVT, most had documented long VA intervals ( Table 1 ).
| Demographics | Value |
|---|---|
| Gestational age at diagnosis (weeks) | |
| Mean | 25 |
| Range | 17–35 |
| Hydrops | 8 (38%) |
| Type | |
| Atrial flutter | 5 (24%) |
| Supraventricular tachycardia | 16 (76%) |
| Short ventriculoatrial interval | 2 |
| Long ventriculoatrial interval | 7 |
| Equal intervals | 4 |
| Indeterminate intervals | 3 |
| Medication | |
| Sotalol alone | 9 (43%) |
| Sotalol with digoxin | 12 (57%) |
| Sotalol with other medication ⁎ | 3 (14%) |
⁎ Sotalol and digoxin were discontinued and other antiarrhythmic agents were tried.
The maximum sotalol dose among the treated pregnant women ranged from 80 mg 2 times/day to 240 mg 3 times/day. Of those who received sotalol therapy, 12 also received digoxin, including 8 who presented with fetal hydrops. Of the 4 others who did not have hydrops, 2 were given an oral digoxin load and then maintenance digoxin dose was started 1 to 2 weeks after the onset of sotalol therapy for synergistic control of the tachycardia, because they had a good response to sotalol but still had brief, intermittent episodes of SVT. Two patients received an intravenous load (1 mg total in divided doses) followed by oral maintenance digoxin simultaneously with the sotalol, according to the referring cardiologist’s preference, as did the 8 who presented with hydrops.
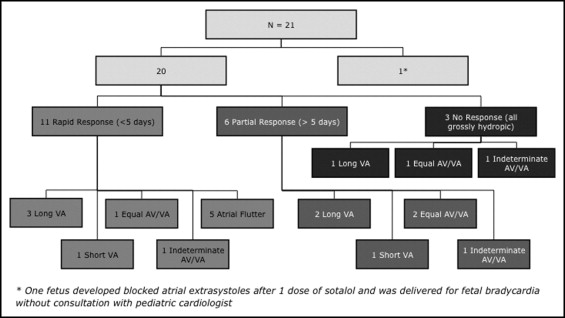
The patients were divided into rapid responders, partial responders, and nonresponders to sotalol therapy ( Figure 3 ). All fetuses with AF were rapid responders; however, the other mechanisms of SVT were represented equally among the responders, partial responders, and nonresponders. One fetus was delivered emergently at 27 weeks for fetal “bradycardia” (average ventricular rate 70 beats/min) after the first dose of sotalol, although on retrospective review of the images, this likely represented blocked atrial extrasystoles. Of the 20 others, 11 had a rapid response, with 10 having full resolution of the tachycardia within 5 days of therapy (median 2), and 1 had significant rate slowing to within the normal sinus rhythm ranges. Six patients (including three with hydrops) were slower to respond or had only a partial response (less frequent SVT in 3 and partial or complete resolution of fluid collections in 3), with a median interval to response of 2 weeks after the onset of therapy. With a desire to completely control the SVT, 3 of these 6 slow/partial responders were given an additional medication (amiodarone in 2 and flecainide in 1), with subsequent conversion. In 1 other with a partial response, the mother chose pregnancy termination. Three grossly hydropic fetuses with SVT showed no response to the sotalol and digoxin combination and died within 1 to 3 days of the onset of treatment with these medications.