There is substantial evidence that the autonomic system plays an important part in the pathogenesis of atrial fibrillation (AF). It appears that, although some patients have a preponderantly sympathetic or vagal overactivation leading to AF, a combined sympathovagal drive is most commonly responsible for AF triggering. The purpose of this hypothesis-generating study was to test whether moxonidine, a centrally acting sympathoinhibitory agent, on top of optimal antihypertensive treatment, can lead to a decrease in AF burden in hypertensive patients with paroxysmal AF. This was a prospective, double-blind, 1-group, crossover study. Hypertensive patients with paroxysmal AF sequentially received treatment with placebo and moxonidine for two 6-week periods, respectively. The change in AF burden (measured as minutes of AF per day in three 48-hour Holter recordings) between the 2 treatment periods was the primary outcome measure. Fifty-six patients (median age 63.5 years, 35 men) were included. During moxonidine treatment, AF burden was reduced from 28.0 min/day (interquartile range [IQR] 15.0 to 57.8) to 16.5 min/day (IQR 4.0 to 36.3; p <0.01). European Heart Rhythm Association symptom severity class decreased from a median of 2.0 (IQR 1.0 to 2.0) to 1.0 (IQR 1.0 to 2.0; p = 0.01). Systolic blood pressure levels were similar in the 2 treatment periods, whereas diastolic blood pressure was lower (p <0.01) during moxonidine treatment. The most frequent complaint was dry mouth (28.6%). No serious adverse events were recorded. In conclusion, treatment with moxonidine, a centrally acting sympathoinhibitory agent, results in reduction of AF burden and alleviation of AF-related symptoms in hypertensive patients with paroxysmal AF.
There is substantial evidence that the autonomic system plays an important part in the pathogenesis of atrial fibrillation (AF). Although some patients have a preponderantly sympathetic or vagal overactivity leading to AF, a combined sympathovagal activation is most commonly responsible for AF triggering. Additional evidence that modulation of the autonomic system and its sympathetic limb in particular, can be of therapeutic interest in AF has been provided by experimental and clinical studies, which showed that renal sympathetic denervation (an intervention that results in suppression of central sympathetic tone) leads to a significant reduction in atrial vulnerability to AF induction and postablation AF recurrence, respectively. The present study tests the hypothesis that modulation of central nervous sympathetic activation by administration of moxonidine, a centrally acting sympathoinhibitory agent, can lead to a decrease in AF burden in hypertensive patients with paroxysmal AF.
Methods
This was a prospective, double-blinded, single-group, crossover study. The study population included hypertensive patients with symptomatic paroxysmal AF. At least 5 minutes of AF per 24 hours in the baseline Holter recording were required for a patient to be considered eligible. Hypertension was defined, for the purposes of the present study, as known treated hypertension or as newly diagnosed hypertension (arterial pressure >140/90 mm Hg in >2 measurements on 2 different days). Exclusion criteria were age <25 or >80 years, left atrial volume >80 ml, known hypersensitivity to moxonidine, sick sinus syndrome or sinoatrial block or conduction abnormalities, bradycardia (<50 beats/min at rest), estimated glomerular filtration rate <30 ml/min/1.73 m 2 , history of angioneurotic edema, impaired left ventricular function (ejection fraction <0.40), stable or unstable angina pectoris, intermittent claudication or known peripheral artery disease, Parkinson’s disease, epileptic disorders, glaucoma, history of depression, pregnancy or lactation, and inability or unwillingness to adhere to standard treatment or to provide consent. The protocol was approved by the Institutional Review Board. All patients provided informed consent. Eligible patients who provided consent were entered in a run-in period of 2 months during which previous treatment was stabilized and antihypertensive treatment was optimized. After the run-in phase, all patients received the study treatment for two 6-week periods: 1 period of active treatment with moxonidine and 1 period of placebo treatment. The patients were randomized as to the sequence of treatments ( Figure 1 ). Moxonidine was started at a dosage of 0.2 mg/day and was increased to 0.4 mg/day after 3 weeks, if well tolerated. If hypotension ensued, it was managed by reducing the doses of other antihypertensives.
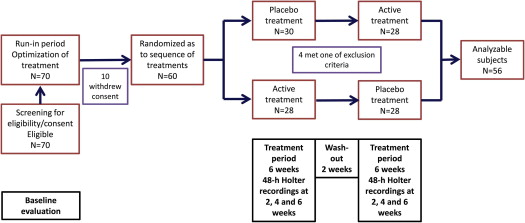
Patient clinical details, symptom severity, and laboratory and echocardiographic parameters were recorded at baseline and on each subsequent visit (visits were scheduled at the end of the second, fourth, and sixth week of each treatment period). Forty-eight-hour Holter recordings were obtained at these time points. The 17-item Hamilton depression rating scale was administered in the last visit of each treatment period.
Patients who failed to present for >1 Holter recordings during each treatment period or who had their antiarrhythmic treatment changed during the study treatment periods were excluded from the analysis. Patient adherence to treatment was checked with pill counts of the drug containers returned by the patients on each study visit. All patients received anticoagulation treatment according to the current guidelines.
The primary outcome measure was AF burden, defined as the minutes of AF per day in the three 48-hour Holter recordings in each treatment phase. Secondary outcome measures were the number of AF episodes per day (AF runs had to be separated by >1 minute of sinus rhythm to be considered as separate episodes of AF), European Heart Rhythm Association symptom severity class, and the 17-item Hamilton depression rating scale score. Monitoring of adverse events focused on xerostomia, gastrointestinal manifestations, headaches, depressive symptoms, and sleep disorders. Procedure implementation and data entry were carried out by researchers who were blinded as to the current patient treatment assignment.
Because of the relatively small sample size, exclusively nonparametric methods were used for analysis. Continuous variables were expressed as median (interquartile range) and compared using nonparametric tests (Wilcoxon’s and Mann-Whitney U test for pairwise and unpaired comparisons, respectively). Categorical variables were expressed as percentages and counts and compared using Fisher’s exact test. Spearman’s correlation index was used to test for correlations between continuous variables. SPSS 17 software package was used (SPSS Inc., Chicago, Illinois). p Values <0.05 (2 sided) were considered as indicative of statistical significance.
Results
Patient characteristics are listed in Table 1 . Most patients had known treated hypertension (71%), whereas the rest were found to be hypertensive as part of the screening procedures. From 70 initially eligible patients, 60 entered the treatment phases of the study, 4 of whom failed to present for >1 Holter recordings per treatment period and were excluded from the analysis ( Figure 1 ).
| Feature | Value |
|---|---|
| Age (yrs) | 63.5 (61.0–68.8) |
| Men | 35 (63) |
| Body mass index (kg/m 2 ) | 26.0 (24.3–30.0) |
| Smokers | 22 (39) |
| Hypertension (by history) | 40 (71) |
| Newly diagnosed hypertension | 16 (29) |
| Diabetes mellitus | 17 (30) |
| AF duration (yrs) | 3.8 (1.6–7) |
| Left ventricular ejection fraction (%) | 55.0 (49.3–60.0) |
| Left atrial volume (ml) | 62 (57–68) |
| Systolic blood pressure (mm Hg) | 143 (136–148) |
| Diastolic blood pressure (mm Hg) | 83 (76–88) |
| European Heart Rhythm Association class | 2.0 (1.0–2.0) |
| AF burden (min/day) | 28.0 (12.5–51.8) |
| AF episodes (no/day) | 3 (2–3) |
| 17-item Hamilton depression rating scale score | 6.0 (4.0–7.8) |
| Treatment | |
| β Blocker | 24 (43) |
| Angiotensin-converting enzyme inhibitor/Angiotensin receptor blocker | 44 (79) |
| Calcium channel blocker | 36 (64) |
| Antiarrhythmic treatment | 28 (50) |
| Amiodarone | 8 (14) |
| Propafenone | 10 (18) |
| Sotalol | 10 (18) |
AF burden was 41% less during the moxonidine treatment phase, compared with the placebo treatment period ( Table 2 ). The difference in the AF burden between the 2 treatment periods was significant even if an independent (unpaired) comparison was performed ( Figure 2 ). AF burden reduction was not related to gender (p = 0.52); age and duration of AF history were also not related to the AF burden difference between placebo and moxonidine treatment (p = 0.29 and 0.93, respectively). Although diastolic blood pressure levels were significantly lower in the moxonidine treatment period, the reduction in diastolic blood pressure was not associated to the reduction in AF burden (p = 0.72).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


