Fig. 10.1
Pathway of endothelial dysfunction and atherosclerosis
The role of endothelial dysfunction in the progression of atherosclerosis and cardiovascular events has been shown in several clinical studies. Endothelial dysfunction predicted progression of carotid artery intima-media thickness in 213 middle-aged civil servants and 618 post-menopausal women, and predicted future cardiovascular events even after adjustment for traditional Framingham cardiovascular risk factors in 3,026 multi ethnic American males and 2,264 post-menopausal Italian women [11–14].
Endothelial Dysfunction in CKD
Endothelial function is highly abnormal in patients with kidney failure. Endothelial dysfunction starts in the very early stages of CKD, progresses with deteriorating kidney failure, is worst on dialysis, and partially improves with kidney transplantation [15]. Unlike in the general population, the evidence of endothelial dysfunction as a risk factor for cardiovascular disease is weak. In a small study with 304 CKD 1–5 patients flow mediated dilation predicted cardiovascular events [16].
Brachial artery flow mediated dilatation is a non-invasive, cheap, established method of determination of endothelial function; it is relatively easily measured by experienced operators [17]. It has been used in CKD patients to test the impact of a variety of interventions such as statins, ACE inhibitors, angiotensin receptor blockers, allopurinol, rosiglitazone, vitamin C, folic acid and sevelemar [18–23]. It is important to note that any changes in endothelial function can be very short lasting. For example, intravenous iron in haemodialysis patients causes endothelial dysfunction within 15–60 min after administration which normalises after 4 h [24].
Mechanism of Effect of Vitamin D on Endothelial Function
The effect of vitamin D on endothelial function has been extensively investigated in the laboratory with in-vivo and in-vitro [25]. Vitamin D modulates endothelial cell function via both genomic transcription and other non-genomic pathways. Endothelial cells are capable of enzymatically activating 25 hydroxy vitamin D to 1,25 dihydroxy vitamin D and hence can respond serum 25 hydroxy vitamin D in severe CKD, where renal activation is significantly impaired [26].
In the endothelial cell vitamin D increases nitric oxide (NO) availability by its possible non-genomic action on intracellular kinases P38, protein kinase B and extracellular signal related kinases (ERK) [27]. This action is mediated via the interaction of vitamin D with vitamin D receptor (VDR) and subsequent phosphorylation of the kinases. Vitamin D reduces reactive oxygen species (ROS) generation via non-genomic suppression of the p22 (phox) subunit of NADPH oxidase [28]. The non-inflammatory effects of vitamin D in endothelial cells are mediated by suppression by gene transcription, via inhibition of NF κβ, of cytokines IL6, IL8, RANTES and cell adhesion molecules ICAM-1, PECAM-1, VCAM-1 and E Selectin [29, 30]. See Fig. 10.2.
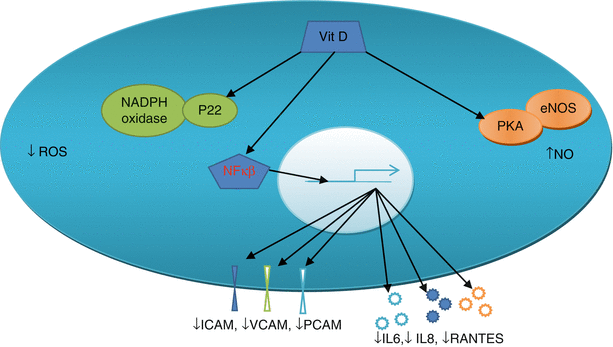

Fig. 10.2
Vitamin D effects on endothelial cell. Vitamin D in the endothelial cell increases nitric oxide (NO) via its non-genomic action on the eNOS system (in orange) and decreases production of reactive oxygen species (ROS) by its non-genomic action on p22 and NADPH oxidase system (green). It decreases the production of ICAM, VCAM and PCAM (dark blue, green and light blue cylinders) and IL6, IL8, RANTES (light blue, dark blue and orange stars) via genomic action and NFκβ
Impact of Vitamin D on Endothelial Function in the Non-CKD Population
Several studies have tested the impact of vitamin D and endothelial cell function in non CKD patients; as summarised Table 10.1 [31–43]. The dose, preparation and duration of therapy varied between the studies, as did the baseline vitamin D concentrations. Endothelial function improved with the administration of vitamin D3 300,000 IU once a month for 3 months in healthy volunteers and with 60,000 IU monthly for 4 months in African Americans [41]. A single dose of 100,000 IU Vitamin D2 was able to improve endothelial function over 2 months in two different studies in patients with diabetes and stroke [42, 43]. The changes in endothelial function 4 months after vitamin D therapy in the post stroke patients were not significant, indicating that the effects were short lasting without on-going supplementation.
Table 10.1
Clinical trials of vitamin D supplementation on endothelial dysfunction in adult patients
Author/ref. | n | Duration (months) | Vitamin D use | Inclusion criterion change in vit. D | Patient type | Outcome |
|---|---|---|---|---|---|---|
Stricker et al. [31]a | 62 | 1 | 100,000 once D3 | <30 ng/ml | PVD | No change |
16 to 24 ng/ml | ||||||
Gepner et al. [32]a | 114 | 4 | 2500/day × 4 months D3 | >10 <60 ng/ml | Post menopause | No change |
31 to 46 ng/ml | ||||||
Witham et al. [34] | 75 | 6 | 100,000 × 3 D3 | <100 nmol/L | Post MI | No change |
44 to 71 nmol/L | ||||||
Longenecker et al. [36] | 45 | 3 | 4000/day × 3 months D3 | <20 ng/ml | HIV patients | No change |
5 ng/ml rise | ||||||
Witham et al. [37]a | 61 | 4 | 100,000 or 200,000 D2 | <100 nmol/l | Diabetes | No change |
Sokol et al. [38]a | 90 | 3 | 50,000/week × 12 D2 | <20 ng/ml | CAD | No change |
13 to 40 ng/ml | ||||||
Witham et al. [33]a | 50 | 2 | 100,000 D3 | <75 nmol/L | South Asian women | No change |
27 to 43 nmol/L | ||||||
Yiu et al. [39]a | 50 | 3 | 5,000/day × 12 weeks D3 | <30 ng/ml | Diabetes | No change |
21 to 55 ng/ml | ||||||
Tarcin et al. [40] | 23 | 3 | 300,000b × 3 D3 | <25 nmol/L | Healthy volunteers | FMD improved |
20 to 116 nmol/L | ||||||
Harris et al. [41]a | 45 | 4 | 60,000/month × 4 D3 | 34 ± 38 nmol/L | African Americans | FMD improved |
34 to 101 nmol/l | ||||||
Witham et al. [42]a | 58 | 4 | 100,000 D2 | <75 nmol/L | Post stroke | FMD improved |
38 to 54 nmol/L | ||||||
Sugden et al. [43]a | 34 | 2 | 100,000 D2 | <50 nmol/L | Diabetes | FMD improved |
38 to 53 nmol/L | ||||||
Dreyer et al. [44]a | 38 | 6 | 350,000 D2 | <30 nmol/L | CKD 3,4 (not diabetic) | Micro-circulation improved |
~90 nmol/L |
Several other studies have failed to show any improvement of endothelial function with vitamin D supplementation [31–39]. Some studies used comparatively lower doses of vitamin D, and others included patients who were not vitamin D deficient or insufficient. However the reason for failure of improvement of endothelial function were not always evident.
Clinical Studies of Vitamin D and Endothelial Function in CKD
Pre-intervention studies in kidney failure patients have examined the relationship between vitamin D deficiency and endothelial function. Vitamin D deficiency has been associated with endothelial dysfunction in both dialysis and pre-dialysis patients. In 52 prevalent dialysis patients flow mediated dilatation (FMD) was directly associated with serum 25 hydroxy vitamin D and 1,25 dihydroxy Vitamin D concentrations [45]. Similarly, in 50 CKD patients decreasing 25 hydroxy Vitamin D levels were associated with decreasing FMD [46].
Very few studies have tested the impact of supplementation of vitamin D on endothelial function in CKD patients. The use of the vitamin D agonist Paricalcitol in two different doses (1 and 2 μg/day) was able to reduce albuminuria and, at higher dose CRP in 24 CKD 2–3 patients; but failed to change FMD [47]. The changes in FMD with placebo, 1 and 2 μg/day was −0.1 % (95 % CI −2.9 to 2.7; p = 0.95); 0.4 % (−2.6 to 3.4; p = 0.79) and 0.3 % (−3.4 to 4.0; p = 0.87) respectively. There was no change in clinic blood pressure and 24 h BP. The baseline PTH concentrations were not significantly elevated and did not change with therapy.
In another study with 26 vitamin D insufficient/deficient patients with CKD stages 3 and 4, two doses of 300,000 cholecalciferol for 16 weeks increased Vitamin D (43 ± 16 to 84 ± 29 nmol/L p < 0.001) decreased PTH (10.8 ± 8.6 to 7.4 ± 4.4 pmol/L, p = 0.001) and improved FMD (3.1 ± 3.3 to 6.1 ± 3.7 %, p < 0.001) [48]. Cholecalciferol therapy improved endothelial cell blood biomarkers E selection, ICAM-1 and VCAM-1. There was no change in blood pressure. Although Vitamin D deficiency is associated with endothelial dysfunction in pre-dialysis and dialysis dependent CKD patients, any benefit of supplementation is not established and randomised controlled trials in CKD patients are required.
Dreyer’s study [44] ] involved 38 subjects (CKD stage 3,4 non diabetic) 20 of whom received ergocalciferol and 18 received placebo. After 6 months, there was a significant improvement in the ergocalciferol-treated group in endothelium dependent microcirculatory vasodilatation after iontophoresis of acetylcholine (p = 0.03) and also there was a reduction seen in tissue advanced glycation end products (p = 0.03). There were no changes in sublingual microcirculatory parameters. Pulse pressure (p = 0.01), but not aortic pulse wave velocity, was reduced. There were no significant changes in bone mineral parameters (curious not to see a PTH reduction), brachial artery blood pressure, or left ventricular mass index suggesting that ergocalciferol improved endothelial function independently of these parameters (or that the effect is too small to detect in a small study group such as here). In parallel ex vivo experiments, expression of endothelial nitric oxide synthase and activity were increased in human endothelial cells in a dose dependent manner.
Hypertension in Chronic Kidney Disease
Hypertension, predominantly systolic, in CKD is almost universal and is the end result of multiple pathophysiologic mechanisms. The activation of the renin-angiotensin and sympathetic nervous systems, fluid retention, endothelial dysfunction, and central arterial stiffness all play major roles in elevating the blood pressure. Hypertension is the most common reversible cardiovascular risk factor for both cardiovascular events and progression of kidney failure in patients with CKD. It is often difficult to reach target blood pressure despite lifestyle modifications and multiple medications. Hence, new methods and new pharmacological agents for the treatment of hypertension in CKD could potentially benefit large numbers of patients suffering from the ill effects of poorly controlled blood pressure.
Mechanism of Effect of Vitamin D on Blood Pressure
Vitamin D can potentially improve endothelial function and lower renin activity and hence lower blood pressure in patients with chronic kidney disease (see Fig. 10.3). Vitamin D affects endothelial function by its genomic transcriptional and other non-genomic actions as discussed above. Vitamin D modulates the renin-angiotensin system by decreasing renin production in experimental animals and humans. This was shown in 15 patients of essential hypertension and hypovitaminosis D, in whom administration of 200,000 IU vitamin D in divided doses over 8 weeks was associated with a reduction of renin and aldosterone levels [49].
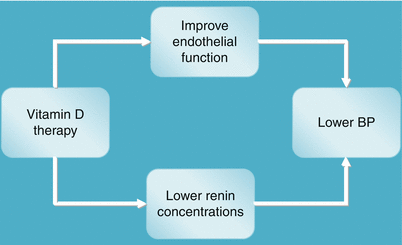

Fig. 10.3
Vitamin D effects on blood pressure. Vitamin D may lower BP via its action improvement of endothelial function and suppressing renin release
Animal experiments suggest 1,25 dihydroxy Vitamin D suppresses renin gene transcription by blocking cyclic AMP-dependant renin gene regulation (see Fig. 10.4) [50]. Cyclic AMP is an intracellular signal for renin production in the juxtaglomerular cells in the kidney. Cyclic AMP, once generated, phosphorylates the transcription factor CREB which with co-activation of CBP/p300 activates the cyclic AMP responsive element (CRE) and promotes renin gene transcription (see Fig. 10.4). The 1,25 dihydroxy vitamin D and vitamin D receptor (VDR) complex interacts with CREB, prevents its binding with CRE, and stops renin gene transcription.
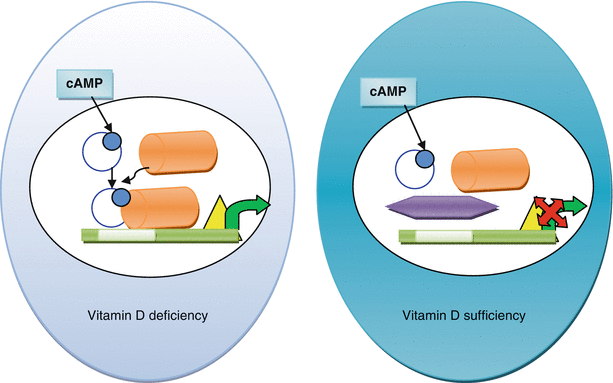





Fig. 10.4
Renin mRNA production in vitamin D deficient and vitamin D sufficient states. Cyclic AMP donates the phosphate (blue dot) to the CREB (blue open circle) and activates it. CRB binds with CBP/p300 (orange cylinder) which in vitamin D deficient state binds to the cAMP responsive element or CRE (white box) on the upstream promoter region of renin gene, activates the RNA polymerase (yellow triangle) and starts transcription of renin mRNA (green arrow). In presence of vitamin D, the vitamin D-VDR (purple hexagon) prevents binding of CRB with CRE
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


