This study was performed to assess the acute intraprocedural effects of transcatheter direct mitral annuloplasty using the Cardioband device on 3-dimensional (3D) anatomy of the mitral annulus. Of 45 patients with functional mitral regurgitation (MR) enrolled in a single arm, multicenter, prospective trial, 22 had complete pre- and post-implant 3D transesophageal echocardiography (TEE) images stored in native data format that allowed off-line 3D reconstruction. Images with the highest volume rate and best image quality were selected for analysis. Multiple measurements of annular geometry were compared from baseline to post-implant using paired t tests with Bonferroni correction to account for multiple comparisons. The device was successfully implanted in all patients, and MR was reduced to moderate in 2 patients, mild in 17 patients, and trace in 3 patients after final device cinching. Compared with preprocedural TEE, postprocedural TEE showed statistically significantly reductions in annular circumference (137 ± 15 vs 128 ± 17 mm; p = 0.042), intercommissural distance (42.4 ± 4.3 vs 38.6 ± 4.4 mm; p = 0.029), anteroposterior distance (40.0 ± 5.4 vs 37.0 ± 5.7 mm; p = 0.025), and aortic-mitral angle (117 ± 8° vs 112 ± 8°; p = 0.032). This study demonstrates that transcatheter direct mitral annuloplasty with the Cardioband device results in acute remodeling of the mitral annulus with successful reduction of functional MR.
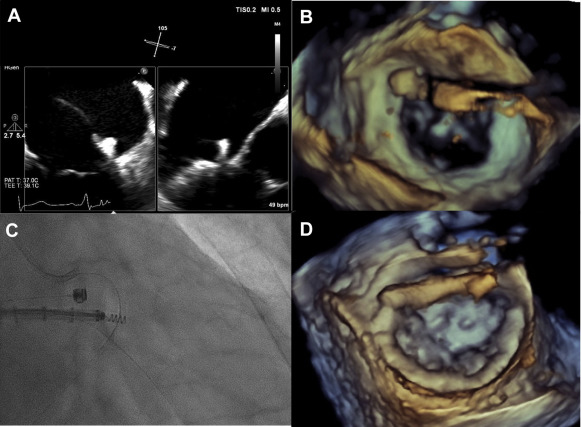
Mitral valve repair remains the standard of care for patients with severe mitral regurgitation (MR). However, because of co-morbidities, poor left ventricular function, and/or age, ∼50% of patients with symptomatic severe MR are not referred for surgery. Thus, percutaneous, catheter-based techniques are being developed to treat high-risk patients with severe MR. MitraClip (Abbott Vascular, Santa Clara, California) is Food and Drug Administration approved for patients with primary MR at high risk for surgery but not for secondary MR. The MitraClip resembles Alfieri’s surgical edge-to-edge technique but does not include mitral annuloplasty, which has been shown to improve the results of surgical edge-to-edge repair. Percutaneous annuloplasty has been reported, typically through the coronary sinus approach. Cardioband (Valtech, Inc., Or Yehuda, Israel) is a transcatheter mitral annuloplasty system that directly anchors to the mitral annulus from the left atrium. After implantation into the beating heart ( Figure 1 ), a Dacron posterior annuloplasty band is cinched under transesophageal echocardiography (TEE) guidance to optimize reduction of MR severity ( Figure 2 ). Here we report the acute intraprocedural effects of the Cardioband device on 3-dimensional (3D) anatomy of the mitral annulus.
Methods
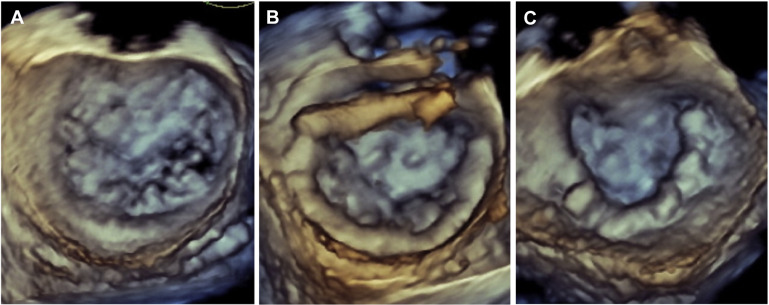
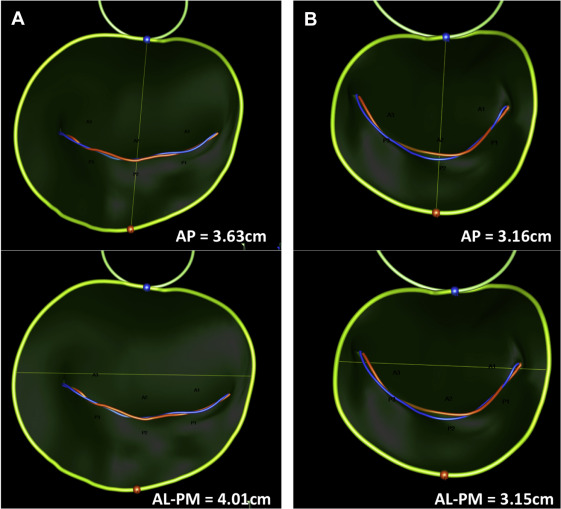
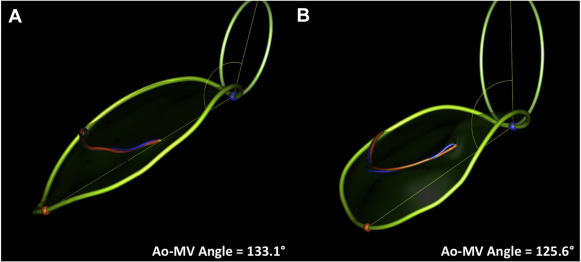
We enrolled 45 patients into a single arm, multicenter, prospective trial to assess the performance and safety of the Cardioband system in patients with functional MR who were deemed by the local heart team to be at excessively high risk for mitral surgery. Details of the study protocol and clinical results at 6 months have been recently reported. Of the 45 patients, 22 patients had complete pre- and post-implant 3D TEE images stored in native data format that allowed off-line 3D reconstruction of mitral annular geometry. Real-time 3D TEE imaging was performed during implantation using either Philips iE33 (Philips Ultrasound, Bothell, Washington) or GE Vivid (GE Healthcare, Horten, Norway). Imaging data sets were analyzed using either Philips QLab Mitral Valve Navigation software or TomTec 4D-mitral valve (MV) Assessment software (TomTec, Munich, Germany). Images acquired on GE Vivid ultrasound platforms were analyzed with the TomTec program; and data sets from Philips iE33 ultrasound systems were analyzed with QLab Mitral Valve Navigation. Images with the highest volume rate and best image quality were selected for baseline and post-implant analysis. Average volume rate across all measured data sets was 15 volumes per second. Data points were manually placed by a single experienced observer (BJR) along anatomic landmarks at end-systole, generating a 3D model of the mitral valve apparatus. The following measurements in 3D space were measured and reported by the software programs: (1) annular circumference in 3D, (2) length of the coaptation line (from anterolateral to posteromedial commissure), (3) intercommissural diameter, (4) anterior posterior diameter, (5) saddle height (distance on the same surface from the highest point of the annulus between the fibrous trigones to the lowest point at the commissures), (6) tenting height (minimal distance between the leaflet coaptation and the mitral annular plane), (7) tenting volume (volume enclosed between the annular plane and mitral leaflets) of the mitral valve at the time of maximal mitral valve closure in systole, (8) anterior leaflet area by 3D echo, (9) length at A2 segment by 3D echo, (10) posterior leaflet area, (11) length at P2, (12) posterior leaflet angle, (13) anterior leaflet angle, and (14) aortic valve-mitral valve angle. Figures 3 and 4 show examples of the reconstructed 3D images.
MR severity was assessed by the core laboratory echocardiographer (PAG) using the integrated approach recommended by the guidelines. Effective regurgitant orifice area, vena contracta width and/or area, color flow jet eccentricity, mitral inflow velocities, and pulmonary venous flow patterns were used to determine MR severity as none/trace, mild, moderate, or severe at baseline and immediately after final device cinching.
Means and SDs of clinical and physiological variables were prospectively collected and tabulated for the 22 patients who had complete baseline and intraoperative 3D TEE measurement profiles ( Table 2 ). Differences between baseline and intraoperative measurements were tested using unadjusted paired t tests to determine significance at the alpha = 0.05 level. Additionally, Bonferroni correction was performed to correct for multiple comparisons. Analyses were performed using SAS 9.3 (Cary, North Carolina).
| Characteristic | |
|---|---|
| Age (years) | 70 ± 7 ∗ |
| Women | 2 (11%) |
| EuroScore II | 6.7 ± 5.7 ∗ |
| LVEF (%) | 30.9 ± 10.7 |
| NYHA Class III or IV | 16 (89%) |
| MR (on intraoperative TEE by Core Lab) | |
| Severe | 14 |
| Moderate | 8 |
| Ischemic functional MR | 12 (67%) |
| Previous CABG | 5 (28%) |
| COPD | 2 (11%) |
| Renal Failure (moderate/severe) | 17 (94%) |
| Atrial fibrillation | 15 (83%) |
| Measurement | Time of measurement | p values | ||
|---|---|---|---|---|
| Baseline | Intra-op | p-value ∗ | Corrected p † | |
| Intercommissural diameter (mm) | 42.3 ± 4.2 | 38.3 ± 4.4 | 0.001 | 0.017 |
| Anterior posterior diameter (mm) | 39.4 ± 5.0 | 36.6 ± 5.5 | 0.002 | 0.025 |
| Saddle height (mm) | 9.0 ± 2.3 | 9.3 ± 2.2 | 0.481 | >0.99 |
| Annular circumference (mm 2 ) | 136 ± 14 | 126 ± 17 | 0.002 | 0.032 |
| Anterior leaflet area (mm 2 ) | 908 ± 142 | 831 ± 194 | 0.023 | 0.391 |
| Posterior leaflet area (mm 2 ) | 661 ± 195 | 569 ± 229 | 0.050 | 0.853 |
| Tenting volume (cm 3 ) | 3.2 ± 2.1 | 2.9 ± 1.6 | 0.263 | >0.99 |
| Anterior leaflet length (mm) | 31.9 ± 2.7 | 30.9 ± 4.6 | 0.236 | >0.99 |
| Posterior leaflet length (mm) | 14.1 ± 3.9 | 12.1 ± 3.5 | 0.014 | 0.243 |
| Anterior leaflet angle (°) | 19.8 ± 5.8 | 18.5 ± 4.4 | 0.413 | >0.99 |
| Posterior leaflet angle (°) | 46.9 ± 12.1 | 50.9 ± 11.8 | 0.078 | >0.99 |
| Tenting height (mm) | 7.2 ± 3.5 | 7.1 ± 2.6 | 0.851 | >0.99 |
| Length of coaptation line (mm) | 43.8 ± 8.2 | 40.9 ± 9.0 | 0.043 | 0.724 |
| Aortic valve-mitral valve angle (°) | 118 ± 8 | 113 ± 8 | 0.002 | 0.032 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


